J Korean Surg Soc.
2009 Jul;77(1):1-6. 10.4174/jkss.2009.77.1.1.
Comparison of E. coli Infiltration between New Synthetic Absorbable Sutures
- Affiliations
-
- 1Department of Surgery, School of Medicine, Daegu Catholic University, Daegu, Korea. hdchae@cu.ac.kr
- KMID: 1464936
- DOI: http://doi.org/10.4174/jkss.2009.77.1.1
Abstract
-
PURPOSE: The proper selection of suture is very important to minimize infection after gastrointestinal anastomosis and closure, which is one of the causes of postoperative complications such as leakage and stricture, etc, in the surgical field. Thus this study focuses on which suture can reduce bacterial infection after surgical operation by comparing in vitro microbial infiltration rates of three synthetic absorbable sutures and that of silk - a relatively absorbable material, using E. coli.
METHODS
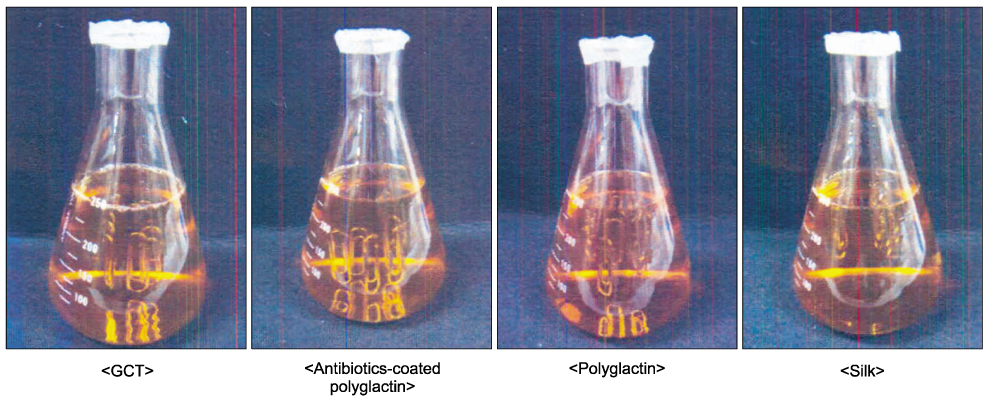
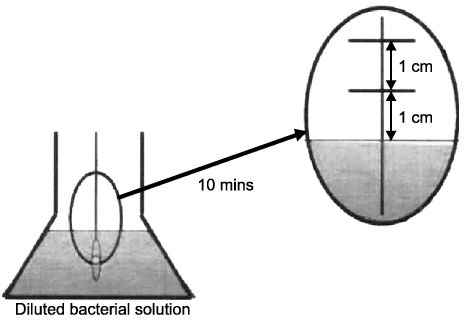
Four different, sterilized kinds of absorbable sutures were used for two experiments. In experiment 1, the cut-off suture was directly applied to the standard method agar plate and cultured for observation. In experiment 2, the cut-off suture was diluted with 1 ml of tryptic soy broth to be smeared and cultured in the standard method agar plate and counted using a spectrophotometer.
RESULTS
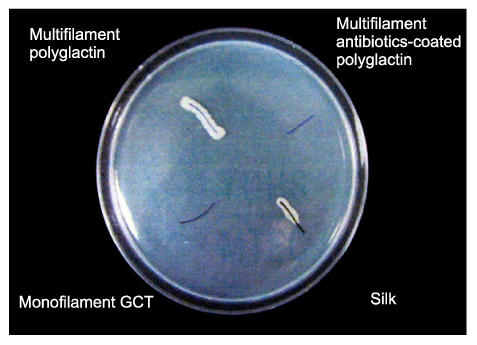
The first experiment revealed that bacterial growth was not observed in the monofilament and antibiotic-coated multifilament sutures, while the other sutures of multifilament structure were invaded by bacteria. In the second experiment, counting and averaging the colony from five plates of each test showed that the number of E. coli of monofilament suture, antibiotics-coated polyglactin, polyglactin and silk were 0+/-0, 39.3+/-14.4, 208.6+/-76.6, 59.4+/-26.7, respectively.
CONCLUSION
Sutures of monofilament structure are believed to be a relatively safe material that can be used for gastrointestinal anastomosis and closure since it has lower bacterial infiltration rates than sutures of multifilament structure.
MeSH Terms
Figure
Reference
-
1. Lo CH, Chen JH, Wu CW, Lo SS, Hsieh MC, Lui WY. Risk factors and management of intra-abdominal infection after extended radical gastrectomy. Am J Surg. 2008. 196:741–745.2. Gomez-Alonso A, Garcia-Criado FJ, Parreno-Manchado FC, Garcia-Sanchez JE, Garcia-Sanchez E, Parreno-Manchado A, et al. Study of the efficacy of coated VICRYL plus antibacterial suture (coated Polyglactin 910 suture with Triclosan) in two animal models of general surgery. J Infect. 2007. 54:82–88.3. Rout WR. Zuidema GD, Yeo CJ, editors. Closure of wound. Shackelford's Surgery of the Alimentary Tract. 2002. 5th ed. Philadelphia: W.B. Saunders;330–347.4. Lee HJ, Kim YH, Yang HK, Lee KU, Choe KJ. The safety and usefulness of synthetic absorbable monofilament, glycoside-epsilon-caprolactone-trimethylene carbonate interpolymer, in gastrointestinal anastomosis and closure. J Korean Gastric Cancer Assoc. 2003. 3:93–96.5. Ray JA, Doddi N, Regula D, Williams JA, Melveger A. Polydioxanone (PDS), a novel monofilament synthetic absorbable suture. Surg Gynecol Obstet. 1981. 153:497–507.6. Bezwada RS, Jamiolkowski DD, Lee IY, Agarwal V, Persivale J, Trenka-Benthin S, et al. Monocryl suture, a new ultra-pliable absorbable monofilament suture. Biomaterials. 1995. 16:1141–1148.7. Rodeheaver GT, Beltran KA, Green CW, Faulkner BC, Stiles BM, Stanimir GW, et al. Biomechanical and clinical performance of a new synthetic monofilament absorbable suture. J Long Term Eff Med Implants. 1996. 6:181–198.8. Katz AR, Mukherjee DP, Kaganov AL, Gordon S. A new synthetic monofilament absorbable suture made from polytrimethylene carbonate. Surg Gynecol Obstet. 1985. 161:213–222.9. Laufman H, Rubel T. Synthetic absorable sutures. Surg Gynecol Obstet. 1977. 145:597–608.10. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992. 20:271–274.11. Irwin ST, Krukowski ZH, Matheson NA. Single layer anastomosis in the upper gastrointestinal tract. Br J Surg. 1990. 77:643–644.12. Burch JM, Franciose RJ, Moore EE, Biffl WL, Offner PJ. Single-layer continuous versus two-layer interrupted intestinal anastomosis: a prospective randomized trial. Ann Surg. 2000. 231:832–837.13. Steele RJ. Continuous single-layer serosubmucosal anastomosis in the upper gastrointestinal tract. Br J Surg. 1993. 80:1416–1417.14. Edmiston CE, Seabrook GR, Goheen MP, Krepel CJ, Johnson CP, Lewis BD, et al. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg. 2006. 203:481–489.15. Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: a review of effectiveness and safety in health care settings. Am J Infect Control. 2000. 28:184–196.16. Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect (Larchmt). 2002. 3:Suppl 1. S79–S87.17. Edmiston C, Schmitt A, Krepel C, Seabrook G. Impact of triclosan-impregnated suture on in vitro adherence of nosocomial surgical pathogens. Am J Infect Control. 2004. 32:E108.18. Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991. 91:Suppl 1. 152S–157S.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of tensile strength of surgical synthetic absorbable suture materials: an in vitro study
- A Comparative Study of the Use of 9-0 PDS and 9-0 Ethilon in Microvascular Anastomosis
- Comparing intra-oral wound healing after alveoloplasty using silk sutures and n-butyl-2-cyanoacrylate
- Retrospective study of superficial wound complication comparison between monofilament absorbable versus monofilament nonabsorbable sutures for skin closure in kidney transplant
- Absorbable Barbed Suture for the Repair of Tendo Calcaneus in Rabbits