J Clin Neurol.
2010 Sep;6(3):148-151. 10.3988/jcn.2010.6.3.148.
Behavioral Changes as the Earliest Clinical Manifestation of Progressive Supranuclear Palsy
- Affiliations
-
- 1Department of Neurology, Myongji Hospital, College of Medicine, Kwandong University, Goyang, Korea. neurohan@kd.ac.kr
- 2Department of Radiology, Myongji Hospital, College of Medicine, Kwandong University, Goyang, Korea.
- 3Department of Pathology, Myongji Hospital, College of Medicine, Kwandong University, Goyang, Korea.
- 4Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- 5Department of Neurology, Hallym University, Ilsong Institute of Life Science, Pyongchon, Korea.
- KMID: 1462851
- DOI: http://doi.org/10.3988/jcn.2010.6.3.148
Abstract
- BACKGROUND
The clinical and pathological heterogeneity of progressive supranuclear palsy (PSP) is well established. Even with a well-defined clinical phenotype and a thorough laboratory workup, PSP can be misdiagnosed, especially in its early stages.
CASE REPORT
A 52-year-old woman, who we initially diagnosed with a behavioral variant of frontotemporal dementia developed parkinsonian features, which then progressed to gait instability and gaze abnormality.
CONCLUSIONS
We report herein a pathologically confirmed case of PSP presenting with behavioral changes including agitation and irritability, which eventually led to the cardinal symptoms of progressive supranuclear palsy.
MeSH Terms
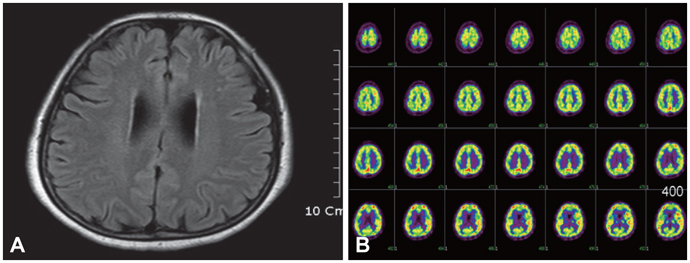
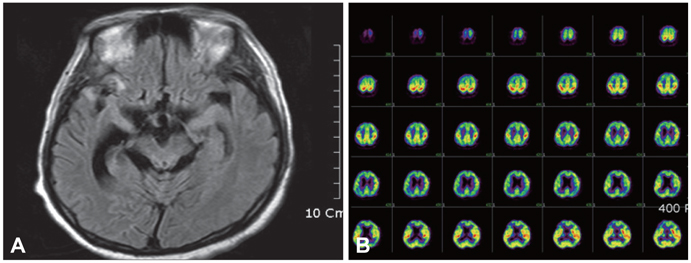
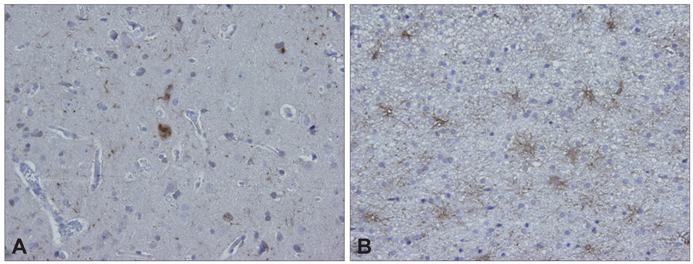
Figure
Reference
-
1. Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996. 47:1–9.
Article2. Pearce JM. Progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): a short historical review. Neurologist. 2007. 13:302–304.
Article3. Kang Y, Na DL, Hahn S. A validity study on the Korean. Mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997. 15:300–308.4. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001. 19:585–591.5. Cummings JL. The Neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology. 1997. 48:S10–S16.
Article6. Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. 1996. 47:1184–1189.
Article7. Grafman J, Litvan I, Gomez C, Chase TN. Frontal lobe function in progressive supranuclear palsy. Arch Neurol. 1990. 47:553–558.
Article8. Trzepacz PT, Murcko AC, Gillespie MP. Progressive supranuclear palsy misdiagnosed as schizophrenia. J Nerv Ment Dis. 1985. 173:377–378.
Article9. Murphy MA, Friedman JH, Tetrud JW, Factor S. Neurodegenerative disorders mimicking progressive supranuclear palsy: a report of three cases. J Clin Neurosci. 2005. 12:941–945.
Article10. Williams DR, de Silva R, Paviour DC, Pitman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005. 128:1247–1258.
Article11. Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006. 66:41–48.
Article12. Wakabayashi K, Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2004. 24:79–86.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Progressive Supranuclear Palsy with Schizophrenic Symptoms
- Progressive Supranuclear Palsy with Predominant Cerebellar Ataxia
- Progressive Supranuclear Palsy Presenting as Primary Progressive Aphasia
- Probable Creutzfeldt-Jakob Disease Presenting as Progressive Supranuclear Palsy
- Striopallidodentate Calcification and Progressive Supranuclear Palsy-Like Phenotype in a Patient with Idiopathic Hypoparathyroidism