J Clin Neurol.
2010 Dec;6(4):189-195. 10.3988/jcn.2010.6.4.189.
Zonisamide Changes Unilateral Cortical Excitability in Focal Epilepsy Patients
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sbhong@skku.edu
- KMID: 1462805
- DOI: http://doi.org/10.3988/jcn.2010.6.4.189
Abstract
- BACKGROUND AND PURPOSE
To evaluate changes in cortical excitability induced by zonisamide (ZNS) in focal epilepsy patients.
METHODS
Twenty-four drug-nasmall yi, Ukrainianve focal epilepsy patients (15 males; overall mean age 29.8 years) were enrolled. The transcranial magnetic stimulation parameters obtained using two Magstim 200 stimulators were the resting motor threshold, amplitude of the motor-evoked potential (MEP), cortical silent period, short intracortical inhibition, and intracortical facilitation. These five transcranial magnetic stimulation parameters were measured before and after ZNS, and the findings were compared.
RESULTS
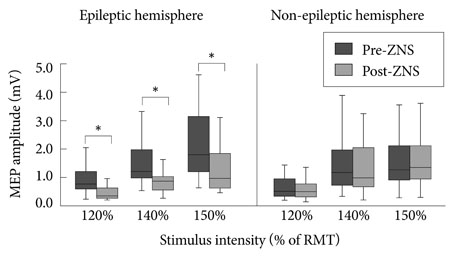
All 24 patients were treated with ZNS monotherapy (200-300 mg/day) for 8-12 weeks. After ZNS, MEP amplitudes decreased (-36.9%) significantly in epileptic hemispheres (paired t-test with Bonferroni's correction for multiple comparisons, p<0.05), whereas the mean resting motor threshold, cortical silent period, short intracortical inhibition, and intracortical facilitation were unchanged (p>0.05). ZNS did not affect cortical excitability in nonepileptic hemispheres.
CONCLUSIONS
These findings suggest that ZNS decreases cortical excitability only in the epileptic hemispheres of focal epilepsy patients. MEP amplitudes may be useful for evaluating ZNS-induced changes in cortical excitability.
Figure
Reference
-
1. Joo EY, Hong SB, Han HJ, Tae WS, Kim JH, Han SJ, et al. Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy. Brain. 2005. 128:1802–1810.
Article2. Lee HW, Hong SB, Tae WS. Opposite ictal perfusion patterns of subtracted SPECT. Hyperperfusion and hypoperfusion. Brain. 2000. 123:2150–2159.
Article3. Luders HO, Engle J, Munari C. Engel J, editor. General principles. Surgical Treatment of the Epilepsies. 1993. New York: Raven Press;137–153.4. Tae WS, Joo EY, Kim JH, Han SJ, Suh YL, Kim BT, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005. 24:101–110.
Article5. Manganotti P, Bongiovanni LG, Zanette G, Turazzini M, Fiaschi A. Cortical excitability in patients after loading doses of lamotrigine: a study with magnetic brain stimulation. Epilepsia. 1999. 40:316–321.
Article6. Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996. 496:873–881.
Article7. Hallett M, Chokroverty S. Magnetic Stimulation in Clinical Neurophysiology. 2005. Philadelphia: Elsevier.8. Delvaux V, Alagona G, Gérard P, De Pasqua V, Delwaide PJ, Maertens de Noordhout A. Reduced excitability of the motor cortex in untreated patients with de novo idiopathic "grand mal" seizures. J Neurol Neurosurg Psychiatry. 2001. 71:772–776.
Article9. Reutens DC, Berkovic SF, Macdonell RA, Bladin PF. Magnetic stimulation of the brain in generalized epilepsy: reversal of cortical hyperexcitability by anticonvulsants. Ann Neurol. 1993. 34:351–355.
Article10. Werhahn KJ, Lieber J, Classen J, Noachtar S. Motor cortex excitability in patients with focal epilepsy. Epilepsy Res. 2000. 41:179–189.
Article11. Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol. 2007. 61:324–331.
Article12. Brodie MJ, Duncan R, Vespignani H, Solyom A, Bitenskyy V, Lucas C. Dose-dependent safety and efficacy of zonisamide: a randomized, double-blind, placebo-controlled study in patients with refractory partial seizures. Epilepsia. 2005. 46:31–41.
Article13. Faught E, Ayala R, Montouris GG, Leppik IE. Zonisamide 922 Trial Group. Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology. 2001. 57:1774–1779.
Article14. Park SP, Kim SY, Hwang YH, Lee HW, Suh CK, Kwon SH. Long-term efficacy and safety of zonisamide monotherapy in epilepsy patients. J Clin Neurol. 2007. 3:175–180.
Article15. Sackellares JC, Ramsay RE, Wilder BJ, Browne TR 3rd, Shellenberger MK. Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia. 2004. 45:610–617.
Article16. Sobieszek G, Borowicz KK, Kimber-Trojnar Z, Małtek R, Piskorska B, Czuczwar SJ. Zonisamide: a new antiepileptic drug. Pol J Pharmacol. 2003. 55:683–689.17. Leppik IE. Zonisamide: chemistry, mechanism of action, and pharmacokinetics. Seizure. 2004. 13:Suppl 1. S5–S9. discussion S10.
Article18. Joo EY, Kim SH, Seo DW, Hong SB. Zonisamide decreases cortical excitability in patients with idiopathic generalized epilepsy. Clin Neurophysiol. 2008. 119:1385–1392.
Article19. Eisai Pharmaceuticals Inc. Zonegran Zonisamide Capsules Prescribing Information. 2004. Teaneck, NJ: Eisai Pharmaceuticals Inc..20. Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, et al. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999. 124:447–454.
Article21. Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998. 51:1320–1324.
Article22. Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993. 471:501–519.
Article23. Reis J, Tergau F, Hamer HM, Müller HH, Knake S, Fritsch B, et al. Topiramate selectively decreases intracortical excitability in human motor cortex. Epilepsia. 2002. 43:1149–1156.
Article24. Ito T, Yamaguchi T, Miyazaki H, Sekine Y, Shimizu M, Ishida S, et al. Pharmacokinetic studies of AD-810, a new antiepileptic compound. Phase I trials. Arzneimittelforschung. 1982. 32:1581–1586.25. Taylor CP, McLean JR, Bockbrader HN, Budannan RA, Xarasaua T, Meyazaki M, et al. Meldrum BS, Porter RJ, editors. Zonisamide (AD-810, CI-912). New Anticonvulsant Drugs. 1986. London: John Libbey;277–294.26. Sohn YH, Kaelin-Lang A, Jung HY, Hallett M. Effect of levetiracetam on human corticospinal excitability. Neurology. 2001. 57:858–863.
Article27. Takano K, Tanaka T, Fujita T, Nakai H, Yonemasu Y. Zonisamide: electrophysiological and metabolic changes in kainic acid-induced limbic seizures in rats. Epilepsia. 1995. 36:644–648.
Article28. Akaike K, Tanaka S, Tojo H, Fukumoto S, Imamura S, Takigawa M. Regional accumulation of 14C-zonisamide in rat brain during kainic acid-induced limbic seizures. Can J Neurol Sci. 2001. 28:341–345.
Article29. Futatsugi Y, Riviello JJ Jr. Mechanisms of generalized absence epilepsy. Brain Dev. 1998. 20:75–79.
Article30. Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001. 31:35–45.
Article31. Kito M, Maehara M, Watanabe K. Mechanisms of T-type calcium channel blockade by zonisamide. Seizure. 1996. 5:115–119.
Article32. Suzuki S, Kawakami K, Nishimura S, Watanabe Y, Yagi K, Seino M, et al. Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 1992. 12:21–27.
Article33. Marinas A, Villanueva V, Giráldez BG, Molins A, Salas-Puig J, Serratosa JM. Efficacy and tolerability of zonisamide in idiopathic generalized epilepsy. Epileptic Disord. 2009. 11:61–66.34. Szaflarski JP. Effects of zonisamide on the electroencephalogram of a patient with juvenile myoclonic epilepsy. Epilepsy Behav. 2004. 5:1024–1026.
Article35. Reutens DC, Berkovic SF. Increased cortical excitability in generalised epilepsy demonstrated with transcranial magnetic stimulation. Lancet. 1992. 339:362–363.
Article36. Macdonell RA, King MA, Newton MR, Curatolo JM, Reutens DC, Berkovic SF. Prolonged cortical silent period after transcranial magnetic stimulation in generalized epilepsy. Neurology. 2001. 57:706–708.
Article37. Manganotti P, Zanette G. Contribution of motor cortex in generation of evoked spikes in patients with benign rolandic epilepsy. Clin Neurophysiol. 2000. 111:964–974.
Article38. Gianelli M, Cantello R, Civardi C, Naldi P, Bettucci D, Schiavella MP, et al. Idiopathic generalized epilepsy: magnetic stimulation of motor cortex time-locked and unlocked to 3-Hz spike-and-wave discharges. Epilepsia. 1994. 35:53–60.
Article39. Chen R, Samii A, Caños M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997. 49:881–883.
Article40. Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996. 40:367–378.
Article41. Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996. 109:127–135.
Article42. Inghilleri M, Conte A, Frasca V, Curra' A, Gilio F, Manfredi M, et al. Antiepileptic drugs and cortical excitability: a study with repetitive transcranial stimulation. Exp Brain Res. 2004. 154:488–493.
Article43. Lee HW, Seo HJ, Cohen LG, Bagic A, Theodore WH. Cortical excitability during prolonged antiepileptic drug treatment and drug withdrawal. Clin Neurophysiol. 2005. 116:1105–1112.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Renal stones during treatment with Zonisamide
- Effects of 1 Hz Repetitive Transcranial Magnetic Stimulation on Cortical Excitability and Seizure Reduction in Intractable Neocortical Epilepsy
- The effect of zonisamide in children with refractory epilepsies
- Focal Cortical Dysplasia in Pediatric Epilepsy
- The Effect of Zonisamide in Epileptic Patients