J Korean Soc Radiol.
2010 Apr;62(4):319-326. 10.3348/jksr.2010.62.4.319.
Assessment of Normal-Appearing White Matter Damage in Multiple Sclerosis Using Diffusion Tensor Imaging
- Affiliations
-
- 1Department of Radiology, College of Medicine, The Catholic University of Korea, Korea. violet2@catholic.ac.kr
- KMID: 1460062
- DOI: http://doi.org/10.3348/jksr.2010.62.4.319
Abstract
- PURPOSE
To determine any evidence of damage in normal-appearing white matter (NAWM) tracts in multiple sclerosis (MS) cases using diffusion tensor imaging (DTI).
MATERIALS AND METHODS
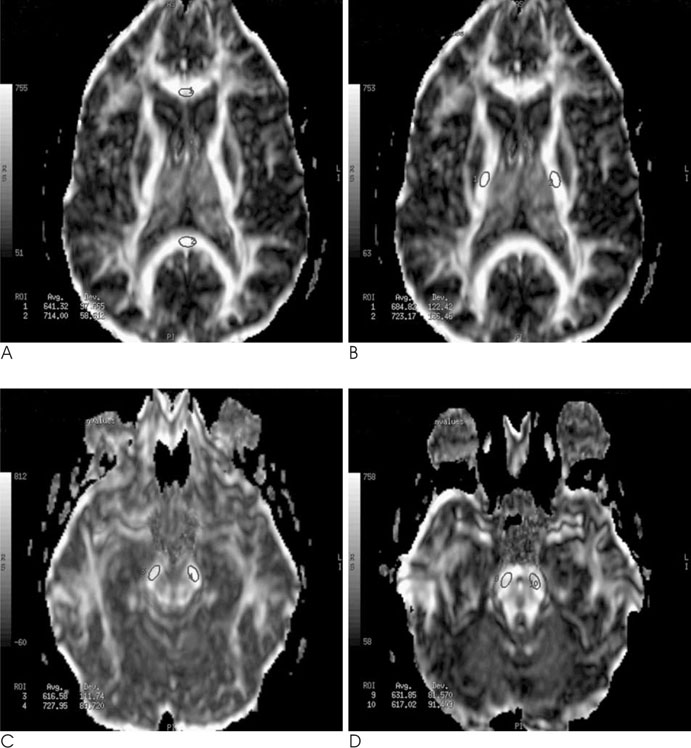
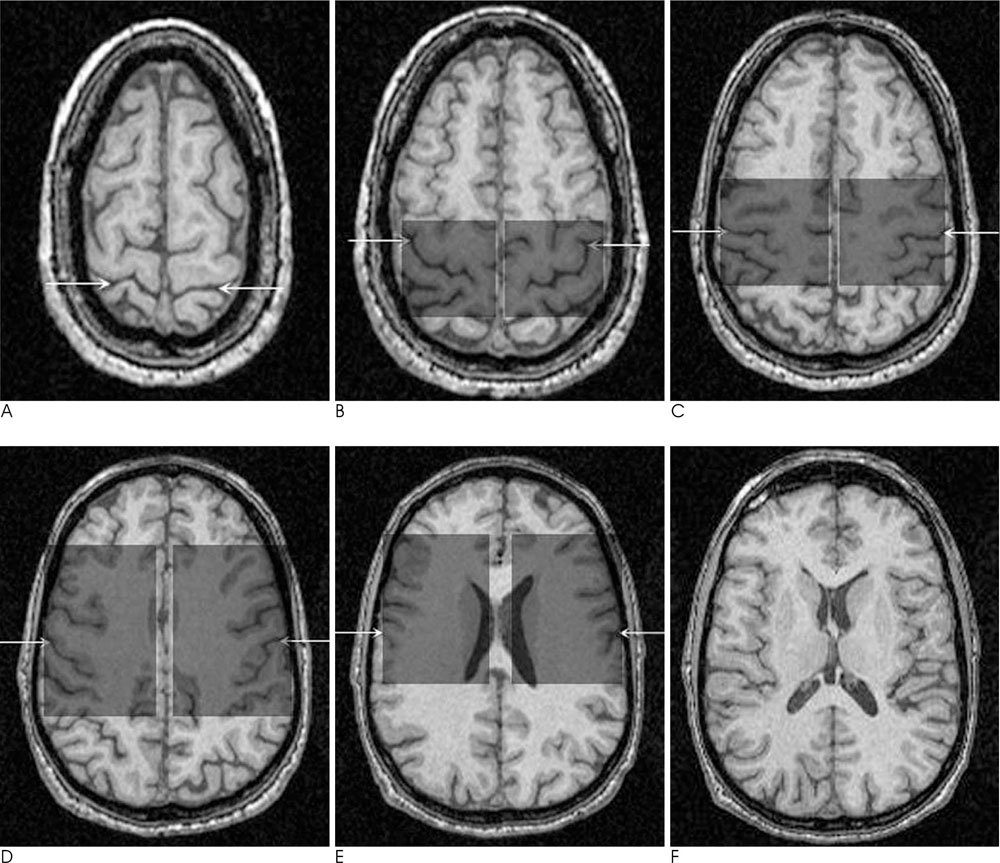
We retrospectively analyzed anisotropy maps derived from DTI studies performed in 16 MS patients and 14 normal controls. Fractional anisotropy (FA) was measured in NAWM tracts: in the genu and splenium of the corpus callosum and at three points along the corticospinal tracts (internal capsule, cerebral peduncle, and pons). In addition, we performed lesion loads using the manual tracing method in the anterior, posterior, corona radiata, and supratentorial of each side. A FA in NAWM tracts was compared between patients and normal controls using the Student t-test. The FA values and lesion load were compared by performing a Spearman rank correlation.
RESULTS
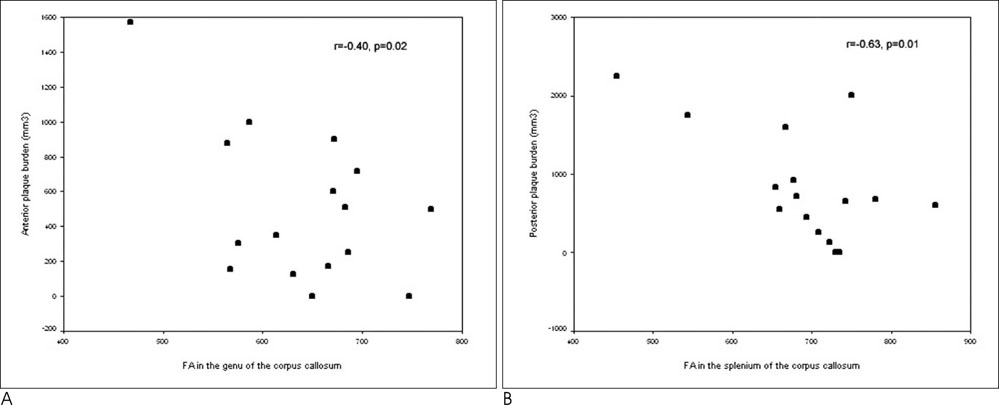
The mean FA values were lower in patients than the controls for the combined genu and splenium (p<0.0001), internal capsule (p=0.03), and cerebral peduncle (p=0.02). Moderate inverse correlations were found between the corpus callosum and the connecting lesion loads (r = -0.40, p = 0.02 for the genu and r=-0.63, p = 0.01 for the splenium). No correlation was found between the FA of the corticospinal tracts and any of the lesion load measurements.
CONCLUSION
We found a statistically significant reduction in the FA values when comparing NAWM tracts from patients with MS those in the normal control group. However, only those in the corpus callosum corresponded with plaque burden. NAWM tract deterioration in the corpus callosum and the corticospinal tracts are likely attributed to several concerted pathologic mechanisms as well as Wallerian degeneration.
MeSH Terms
Figure
Reference
-
1. Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998; 121:3–24.2. McDonald WI, Miller DH, Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992; 18:319–334.3. Barkhof F, Filippi M, Miller DH, Tofts P, Kappos L, Thompson AJ. Strategies of optimizing MRI techniques aimed at monitoring disease activity in multiple sclerosis treatment. J Neurol. 1997; 244:76–84.4. Miller DH, Alberts PS, Barkhof F, Francis G, Frank JA, Hodgkinson S, et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. Ann Neurol. 1996; 39:6–16.5. Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal appearing white matter in multiple sclerosis. Neurology. 1999; 52:1626–1632.6. Waxman SG. Demyelinating diseases: new pathological insights, new therapeutic targets. N Engl J Med. 1998; 338:323–325.7. Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med. 2000; 44:583–591.8. Cercignani M, Iannucci G, Rocca MA, Comi G, Horsfield MA, Filippi M. Pathologic damage in MS assessed by diffusion-weighted and magnetization transfer MRI. Neurology. 2000; 54:1139–1144.9. Nusbaum AO, Tang CY, Wei T, Buchsbaum MS, Atlas SW. Whole-brain diffusion MR histograms differ between MS subtypes. Neurology. 2000; 54:1421–1427.10. Ciccarelli O, Werring DJ, Wheeler-Kingshott CAM, Barker GJ, Parker GJ, Thompson AJ, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001; 56:926–933.11. Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Magnetization transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001; 70:311–317.12. Marinero C, De Stefano N, Iannucci G, Sormani MP, Guidi L, Federico A, et al. Correlates of MS disability assessed in vivo using aggregates of MR quantities. Neurology. 2001; 56:1331–1334.13. Christiansen P, Gideon P, Thomsen C, Stubqaard M, Henriksen O, Larsson HB, et al. Increased water self-diffusion in chronic plaques and in apparently normal white matter in patients with multiple sclerosis. Acta Neurol Scand. 1993; 87:195–199.14. Ciccarelli O, Werring DJ, Barker GJ, Griffin CM, Wheeler-Kingshott CA, Miller DH, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging- evidence of Wallerian degeneration. J Neurol. 2003; 250:287–292.15. Simon JH, Kinkel RP, Jacobs L, Bub L, Simonian N. A Wallerian degeneration pattern in patients at risk for MS. Neurology. 2000; 54:1155–1160.16. Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997; 120:393–399.17. Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transsection in the lesions of multiple sclerosis. N Engl J Med. 1998; 338:278–285.18. Casanova B, Martinez-Bisbal MC, Valero C, Celda B, Marti-Bonmati L, Pascual A, et al. Evidence of wallerian degeneration in normal appearing white matter in the early stages of relapsing-remitting multiple sclerosis; a HMRS study. J Neurol. 2003; 250:22–28.19. Basser P. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995; 8:333–344.20. Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994; 31:394–400.21. Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996; 201:637–648.22. Virta A, Barnett A, Pierpaoli C. Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magn Reson Imaging. 1999; 17:1121–1133.23. Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001; 13:1174–1185.24. Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain. 2000; 123:1845–1849.25. Arnold DL, Matthews PM, Francis GS, O’Connor J, Antel JP. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol. 1992; 31:235–241.26. Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000; 157:267–276.27. Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology. 2002; 222:729–736.28. Guo AC, Jewells VL, Provenzale JM. Analysis of normal-appearing white matter in multiple sclerosis: comparison of diffusion tensor MR imaging and magnetization transfer imaging. AJNR Am J Neuroradiol. 2001; 22:1893–1900.29. Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, et al. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology. 1995; 45:478–482.30. Catalaa I, Grossman RI, Kolson DL, Udupa JK, Nyul LG, Wei L, et al. Multiple sclerosis: magnetization transfer histogram analysis of segmented normal-appearing white matter. Radiology. 2000; 216:351–355.31. Tortorella C, Viti B, Bozzali M, Sormani MP, Rizzo G, Gilardi ME, et al. A magnetization transfer histogram study of normal-appearing brain tissue in MS. Neurology. 2000; 54:186–193.32. Revesz T, Kidd D, Thompson AJ, Barnard RO, McDonald WI. A comparison of the pathology of primary and secondary progressive multiple sclerosis. Brain. 1994; 117:759–765.33. Thompson AJ, Kermode AG, Wicks D, MacManus DG, Kendall BE, Kingsley DP, et al. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol. 1991; 29:53–62.34. Kidd D, Thorpe JW, Kendall BE, Barker GJ, Miller DH, McDonal WI, et al. MRI dynamics of brain and spinal cord in progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996; 60:15–19.35. Barnes D, Munro PM, Youl BD, Prineas JW, McDonald WI. The longstanding MS lesion. A quantitative MRI and electron microscopic study. Brain. 1991; 114:1271–1280.36. Griffin CM, Chard DT, Parker GJ, Barker GJ, Thompson AJ, Miller DH. The relationship between lesion and normal appearing brain tissue abnormalities in early relapsing remitting multiple sclerosis. J Neurol. 2002; 249:193–199.37. DeLuca GC, Ebers GC, Esiri MM. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal tract and sensory tracts. Brain. 2004; 127:1009–1018.38. Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000; 123:1174–1183.39. Patry DW, Li DK, Oger JJ, Kastrukoff L, Koopmans R, Tanton E, et al. Magnetic resonance imaging in the evaluation of clinical trials in multiple sclerosis. Ann Neurol. 1994; 36:S95–S96.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Tensor Imaging Findings of White Matter Changes in First Episode Schizophrenia: A Systematic Review
- Alteration of White Matter Integrity in Dyslexic Children: Case-Control Study
- Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders
- The Usefulness of Diffusion-tensor MR Imaging (DTI) in the Grading of Gliomas
- Brain Diffusion Tensor MR Imaging