J Bacteriol Virol.
2010 Jun;40(2):59-66. 10.4167/jbv.2010.40.2.59.
Anaerobiosis of Pseudomonas aeruginosa: Implications for Treatments of Airway Infection
- Affiliations
-
- 1Department of Microbiology and Brain Korea 21 Project for Medical Sciences, Yonsei University College of Medicine, Seoul, Korea. sangsun_yoon@yuhs.ac
- KMID: 1456268
- DOI: http://doi.org/10.4167/jbv.2010.40.2.59
Abstract
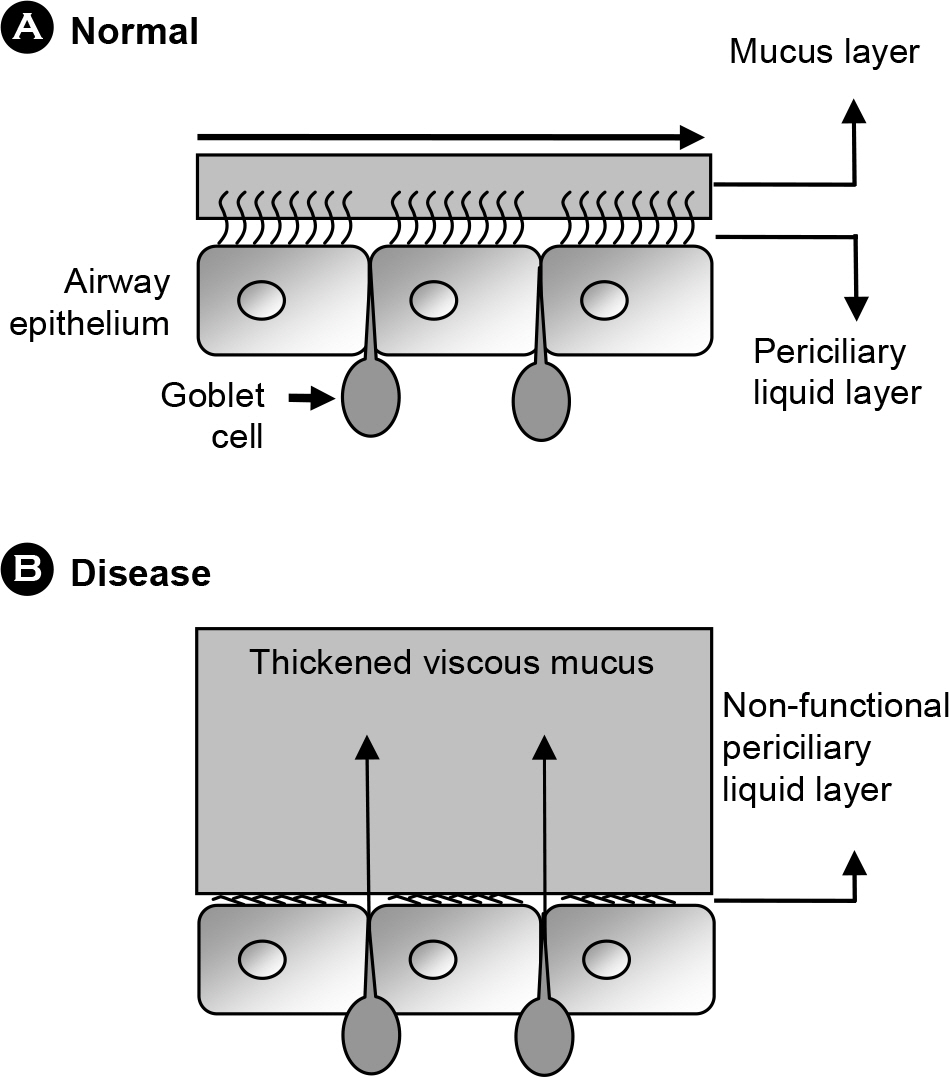
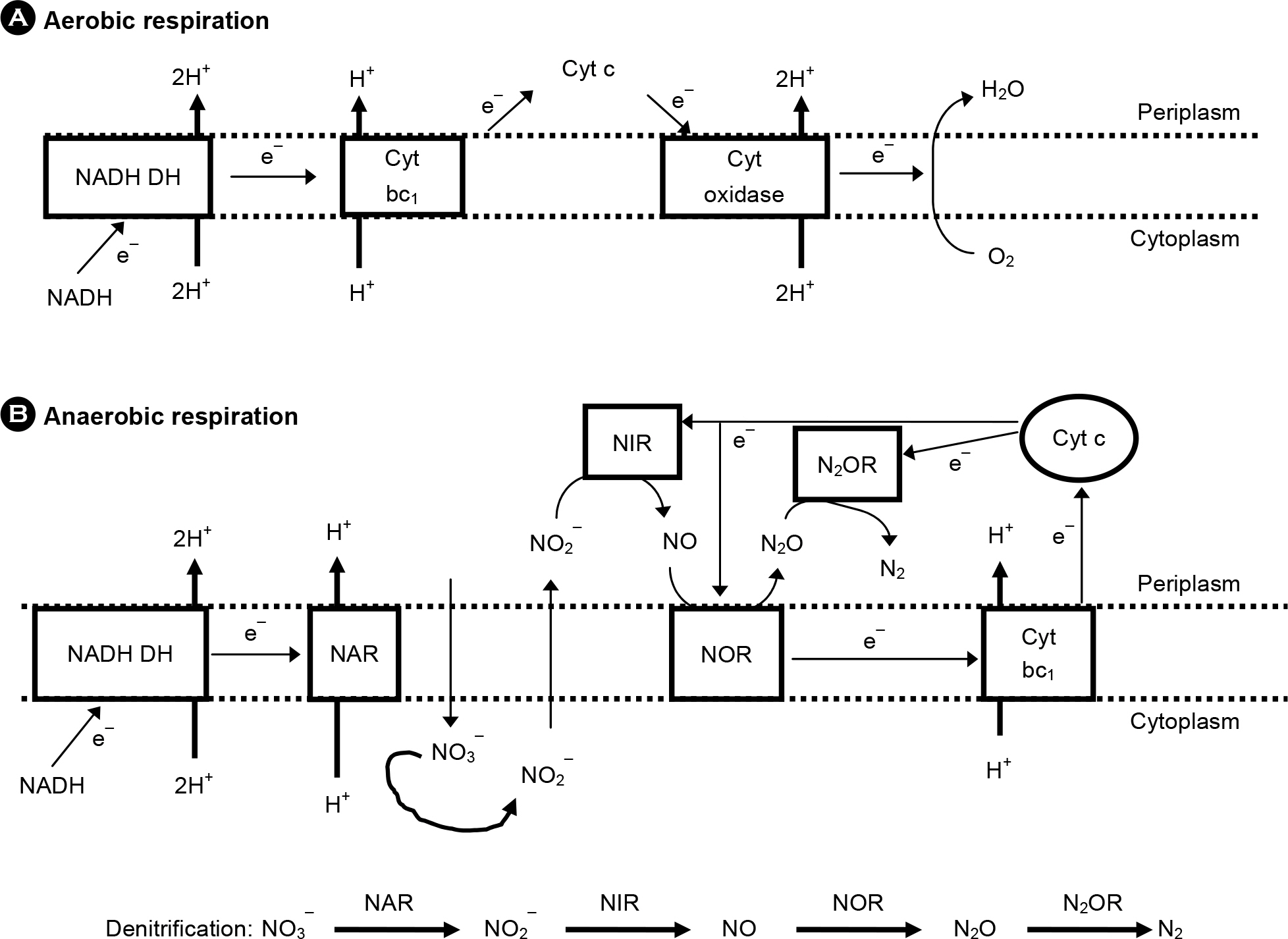
- Pseudomonas aeruginosa, as an opportunistic pathogen, establishes a chronic infection in the respiratory track of patients suffering from pneumonia and bronchiectasis, including cystic fibrosis. Biofilm formation inside the oversecreted mucus layer lining the patient airway and production of virulence factors, a process controlled by quorum sensing, are considered to be the major virulence determinants in P. aeruginosa pathogenesis. Recently, an abnormally thickened mucus layer was proven to be anaerobic. Given the fact that currently used antibiotics are less effective under anaerobic environments, these new findings lead us to change the way we confront P. aeruginosa infection. This article reviews pathological features of patient airways that become susceptible to P. aeruginosa infection and bacterial adaptation that contributes to the prolonged survival inside the patient airway.
MeSH Terms
Figure
Reference
-
1). Yoon SS., Hassett DJ. Chronic Pseudomonas aeruginosa infection in cystic fibrosis airway disease: metabolic changes that unravel novel drug targets. Expert Rev Anti Infect Ther. 2004. 2:611–23.2). Lyczak JB., Cannon CL., Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000. 2:1051–60.3). Yoon SS., Hennigan RF., Hilliard GM., Ochsner UA., Parvatiyar K., Kamani MC, et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell. 2002. 3:593–603.4). Yoon SS., Karabulut AC., Lipscomb JD., Hennigan RF., Lymar SV., Groce SL, et al. Two-pronged survival strategy for the major cystic fibrosis pathogen, Pseudomonas aeruginosa, lacking the capacity to degrade nitric oxide during anaerobic respiration. EMBO J. 2007. 26:3662–72.5). Parsek MR., Tolker-Nielsen T. Pattern formation in Pseudomonas aeruginosa biofilms. Curr Opin Microbiol. 2008. 11:560–6.6). Aparna MS., Yadav S. Biofilms: microbes and disease. Braz J Infect Dis. 2008. 12:526–30.
Article7). Filloux A., Vallet I. Biofilm: set-up and organization of a bacterial community. Med Sci (Paris). 2003. 19:77–83.8). Hassett DJ., Elkins JG., Ma JF., McDermott TR. Pseudomonas aeruginosa biofilm sensitivity to biocides: use of hydrogen peroxide as model antimicrobial agent for examining resistance mechanisms. Methods Enzymol. 1999. 310:599–608.9). Hill D., Rose B., Pajkos A., Robinson M., Bye P., Bell S, et al. Antibiotic susceptabilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. J Clin Microbiol. 2005. 43:5085–90.10). Stewart PS., Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001. 358:135–8.
Article11). Teitzel GM., Parsek MR. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol. 2003. 69:2313–20.12). Jesaitis AJ., Franklin MJ., Berglund D., Sasaki M., Lord CI., Bleazard JB, et al. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol. 2003. 171:4329–39.13). Bonomo RA., Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006. ;43 Suppl. 2:S49–56.14). Wiedemann B., Heisig P. Mechanisms of quinolone resistance. Infection. 1994. ;22 Suppl. 2:S73–9.
Article15). Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J Appl Microbiol. 2002. ;92 Suppl:55S-64S.
Article16). Mariani-Kurkdjian P., Bingen E. Pseudomonas aeruginosa: resistance to antibiotics. Arch Pediatr. 2006. ;13 Suppl. 1:S5–9.17). Lazdunski A., Guzzo J., Filloux A., Bally M., Murgier M. Secretion of extracellular proteins by Pseudomonas aeruginosa. Biochimie. 1990. 72:147–56.18). Heck LW., Alarcon PG., Kulhavy RM., Morihara K., Russell MW., Mestecky JF. Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J Immunol. 1990. 144:2253–7.19). Wretlind B., Pavlovskis OR. The role of proteases and exotoxin A in the pathogenicity of Pseudomonas aeruginosa infections. Scand J Infect Dis Suppl. 1981. 29:13–9.20). Guzzo J., Duong F., Wandersman C., Murgier M., Lazdunski A. The secretion genes of Pseudomonas aeruginosa alkaline protease are functionally related to those of Erwinia chrysanthemi proteases and Escherichia coli alpha-haemolysin. Mol Microbiol. 1991. 5:447–53.21). Pollack M. The role of exotoxin A in pseudomonas disease and immunity. Rev Infect Dis. 1983. ;5 Suppl. 5:S979–84.
Article22). Cryz SJ Jr., Furer E., Sadoff JC., Germanier R., Pastan I., Willingham MC, et al. Use of Pseudomonas aeruginosa toxin A in the construction of conjugate vaccines and immunotoxins. Rev Infect Dis. 1987. 9(Suppl 5):S644–9.23). Berk RS., Brown D., Coutinho I., Meyers D. In vivo studies with two phospholipase C fractions from Pseudomonas aeruginosa. Infect Immun. 1987. 55:1728–30.24). Berka RM., Vasil ML. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol. 1982. 152:239–45.25). Lau GW., Hassett DJ., Ran H., Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004. 10:599–606.26). Antunes MB., Cohen NA. Mucociliary clearance–a critical upper airway host defense mechanism and methods of assessment. Curr Opin Allergy Clin Immunol. 2007. 7:5–10.27). Fokkens WJ., Scheeren RA. Upper airway defence mechanisms. Paediatr Respir Rev. 2000. 1:336–41.
Article28). Umeki S. Primary mucociliary transport failure. Respiration. 1988. 54:220–5.
Article29). Tiddens HA., Donaldson SH., Rosenfeld M., Pare PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol. 2010. 45:107–17.
Article30). Frey HR., Russi EW. Bronchiectasis–current aspects of an old disease. Schweiz Med Wochenschr. 1997. 127:219–30.31). Clarke SW. Management of mucus hypersecretion. Eur J Respir Dis Suppl. 1987. 153:136–44.32). Reynolds HY. Host defense impairments that may lead to respiratory infections. Clin Chest Med. 1987. 8:339–58.
Article33). Fahy JV., Schuster A., Ueki I., Boushey HA., Nadel JA. Mucus hypersecretion in bronchiectasis. The role of neutrophil proteases. Am Rev Respir Dis. 1992. 146:1430–3.
Article34). Ratjen F., Doring G. Cystic fibrosis. Lancet. 2003. 361:681–9.
Article35). Bangel N., Dahlhoff C., Sobczak K., Weber WM., Kusche-Vihrog K. Upregulated expression of ENaC in human CF nasal epithelium. J Cyst Fibros. 2008. 7:197–205.
Article36). Worlitzsch D., Tarran R., Ulrich M., Schwab U., Cekici A., Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002. 109:317–25.37). Meyer KC., Zimmerman J. Neutrophil mediators, Pseudomonas, and pulmonary dysfunction in cystic fibrosis. J Lab Clin Med. 1993. 121:654–61.38). Jagger KS., Robinson DL., Franz MN., Warren RL. Detection by enzyme-linked immunosorbent assays of antibody specific for Pseudomonas proteases and exotoxin A in sera from cystic fibrosis patients. J Clin Microbiol. 1982. 15:1054–8.39). Kerscher S., Drose S., Zickermann V., Brandt U. The three families of respiratory NADH dehydrogenases. Results Probl Cell Differ. 2008. 45:185–222.
Article40). Mulkidjanian AY. Proton translocation by the cytochrome bc1 complexes of phototrophic bacteria: introducing the activated Q-cycle. Photochem Photobiol Sci. 2007. 6:19–34.41). Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K, et al. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995. 269:1069–74.42). Gresser MJ., Myers JA., Boyer PD. Catalytic site cooperativity of beef heart mitochondrial F1 adenosine triphosphatase. Correlations of initial velocity, bound intermediate, and oxygen exchange measurements with an alternating three-site model. J Biol Chem. 1982. 257:12030–8.
Article43). Linnane SJ., Keatings VM., Costello CM., Moynihan JB., O'Connor CM., Fitzgerald MX, et al. Total sputum nitrate plus nitrite is raised during acute pulmonary infection in cystic fibrosis. Am J Respir Crit Care Med. 1998. 158:207–12.
Article44). Jones KL., Hegab AH., Hillman BC., Simpson KL., Jinkins PA., Grisham MB, et al. Elevation of nitro-tyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr Pulmonol. 2000. 30:79–85.
Article45). Costerton JW., Stewart PS., Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999. 284:1318–22.
Article46). O'Toole GA., Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998. 30:295–304.47). Sriramulu DD., Lunsdorf H., Lam JS., Romling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005. 54:667–76.48). Ryder C., Byrd M., Wozniak DJ. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol. 2007. 10:644–8.49). Mah TF., Pitts B., Pellock B., Walker GC., Stewart PS., O'Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003. 426:306–10.50). Sadovskaya I., Vinogradov E., Li J., Hachani A., Kowalska K., Filloux A. High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: the ndvB gene is involved in the production of highly glyceroL-phosphorylated {beta}-(1->3)-glucans, which bind aminoglycosides. Glycobiology. 2010. 20:895–904.51). Xu KD., Stewart PS., Xia F., Huang CT., McFeters GA. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998. 64:4035–9.52). de Kievit TR. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009. 11:279–88.53). Storey DG., Ujack EE., Rabin HR., Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun. 1998. 66:2521–8.54). Pearson JP., Pesci EC., Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997. 179:5756–67.55). Howe TR., Iglewski BH. Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun. 1984. 43:1058–63.56). Hassett DJ., Ma JF., Elkins JG., McDermott TR., Ochsner UA., West SE, et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol. 1999. 34:1082–93.57). Clatworthy AE., Lee JS., Leibman M., Kostun Z., Davidson AJ., Hung DT. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect Immun. 2009. 77:1293–303.58). Papaioannou E., Wahjudi M., Nadal-Jimenez P., Koch G., Setroikromo R., Quax WJ. Quorum-quenching acylase reduces the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model. Antimicrob Agents Chemother. 2009. 53:4891–7.59). Tang HB., DiMango E., Bryan R., Gambello M., Iglewski BH., Goldberg JB, et al. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996. 64:37–43.60). Sawa T., Ohara M., Kurahashi K., Twining SS., Frank DW., Doroques DB, et al. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun. 1998. 66:3242–9.61). Chun CK., Ozer EA., Welsh MJ., Zabner J., Greenberg EP. Inactivation of a Pseudomonas aeruginosa quorumsensing signal by human airway epithelia. Proc Natl Acad Sci U S A. 2004. 101:3587–90.62). Gambello MJ., Iglewski BH. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991. 173:3000–9.63). Pesci EC., Pearson JP., Seed PC., Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997. 179:3127–32.64). Pesci EC., Milbank JB., Pearson JP., McKnight S., Kende AS., Greenberg EP, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999. 96:11229–34.65). Diggle SP., Cornelis P., Williams P., Camara M. 4-quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int J Med Microbiol. 2006. 296:83–91.66). Calfee MW., Coleman JP., Pesci EC. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2001. 98:11633–7.67). McKnight SL., Iglewski BH., Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000. 182:2702–8.68). Jensen V., Lons D., Zaoui C., Bredenbruch F., Meissner A., Dieterich G. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J Bacteriol. 2006. 188:8601–6.69). D'Argenio DA., Wu M., Hoffman LR., Kulasekara HD., Deziel E., Smith EE, et al. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol. 2007. 64:512–33.70). Hoffman LR., Kulasekara HD., Emerson J., Houston LS., Burns JL., Ramsey BW, et al. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros. 2009. 8:66–70.71). Hoffman LR., Richardson AR., Houston LS., Kulasekara HD., Martens-Habbena W., Klausen M, et al. Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLoS Pathog. 2010. 6:e1000712.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Approach to a vaccine against Pseudomonas aeruginosa
- Overexpression of Efflux Pump in Multiresistant Pseudomonas aeruginosa: How You Will Discover and Treat It?
- Effect of pseudomonas aeruginosa infection on production of IL-2 and IL-6, and other parameters of immunocompetency in mice
- Fatal Necrotizing Fasciitis Due to Pseudomonas aeruginosa After Vaccination : A Case Report
- A Case of Pseudomonas aeruginosa Abscess Developing after Gluteal Intramuscular Injection