J Clin Neurol.
2011 Mar;7(1):25-30. 10.3988/jcn.2011.7.1.25.
SLC25A4 and C10ORF2 Mutations in Autosomal Dominant Progressive External Ophthalmoplegia
- Affiliations
-
- 1Department of Neurology, Pusan National University Yangsan Hospital, Yangsan, Korea. dskim@pusan.ac.kr
- 2Medical Research Institute, Pusan National University School of Medicine, Yangsan, Korea.
- 3Department of Neurology, Pusan National Univerdity Hospital, Busan, Korea.
- 4Department of Pathology, Pusan National Univerdity Hospital, Busan, Korea.
- KMID: 1452569
- DOI: http://doi.org/10.3988/jcn.2011.7.1.25
Abstract
- BACKGROUND AND PURPOSE
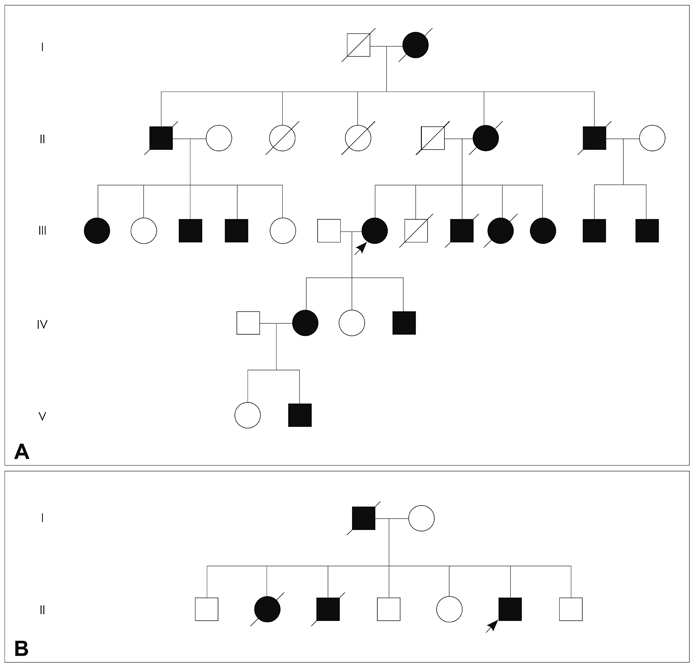
Progressive external ophthalmoplegia (PEO) with Mendelian inheritance is a heterogeneous group of diseases associated with multiple deletions of mitochondrial DNA (mtDNA), which results from the disturbed replication and maintenance of mtDNA secondary to the mutations of nuclear genes including POLG, SLC25A4, C10ORF2, POLG2, OPA1, and RRM2B. The aim of this study was to identify the genetic defects underlying the pathology and clinical features in two Korean kindreds with autosomal dominant PEO.
METHODS
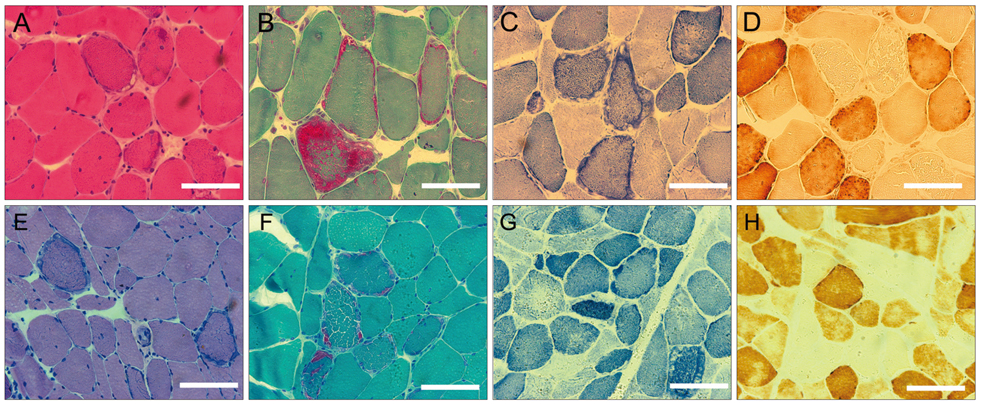
Two pathologically proven PEO patients with a clear autosomal dominant pattern of inheritance were selected. To exclude a large-scale rearrangement, a long-range polymerase chain reaction (PCR) was performed using DNA extracted from biopsied muscle tissue taken from each patient. All coding regions and exon-intron boundaries of POLG, SLC25A4, C10ORF2, and POLG2 were amplified by PCR and directly sequenced.
RESULTS
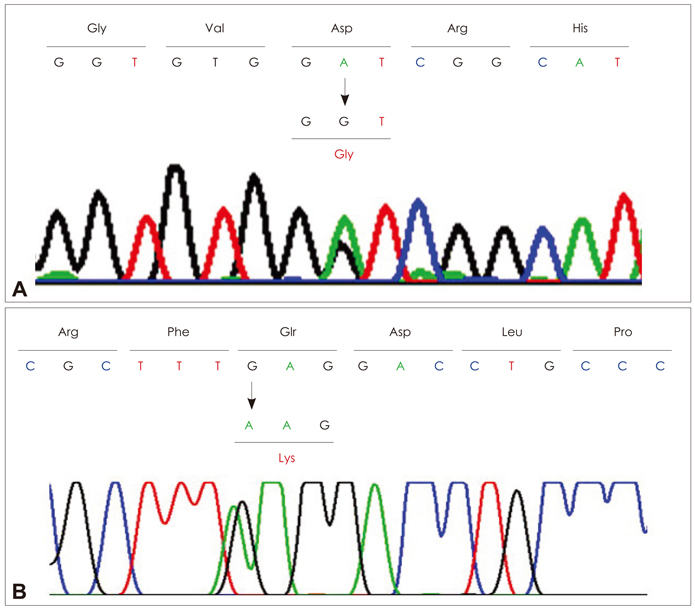
One patient showed multiple deletions of mtDNA on long-range PCR analysis, and two known heterozygous missense mutations in SLC25A4 (p.Asp104Gly) and C10ORF2 (p.Glu479Lys) were identified in each patient. The p.Asp104Gly mutation in SLC25A4 was identified in the patient with an early onset, slowly progressive, pure PEO phenotype, while the p.Glu479Lys mutation in C10ORF2 was identified in the other patient, with a late-onset disease and PEO plus phenotype.
CONCLUSIONS
Two mutations affecting nuclear genes were identified in Korean patients with autosomal dominant PEO. Further studies are necessary to identify the clear pathogenetic mechanisms and establish genotype-phenotype correlations in autosomal dominant PEO.
MeSH Terms
Figure
Reference
-
1. DiMauro S, Hirano M. Pagon RA, Bird TC, Dolan CR, Stephens K, editors. Mitochondrial DNA Deletion Syndromes. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle.2. Mariotti C, Savarese N, Suomalainen A, Rimoldi M, Comi G, Prelle A, et al. Genotype to phenotype correlations in mitochondrial encephalomyopathies associated with the A3243G mutation of mitochondrial DNA. J Neurol. 1995. 242:304–312.
Article3. Zeviani M, Servidei S, Gellera C, Bertini E, DiMauro S, DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989. 339:309–311.
Article4. Kaukonen J, Juselius JK, Tiranti V, Kyttälä A, Zeviani M, Comi GP, et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science. 2000. 289:782–785.
Article5. Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. 2001. 28:223–231.
Article6. Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet. 2001. 28:211–212.
Article7. Longley MJ, Clark S, Yu Wai Man C, Hudson G, Durham SE, Taylor RW, et al. Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia. Am J Hum Genet. 2006. 78:1026–1034.
Article8. Tyynismaa H, Ylikallio E, Patel M, Molnar MJ, Haller RG, Suomalainen A. A heterozygous truncating mutation in RRM2B causes autosomal-dominant progressive external ophthalmoplegia with multiple mtDNA deletions. Am J Hum Genet. 2009. 85:290–295.
Article9. Koh KN, Park SY, Hwang H, Chae JH, Choi JE, Kim KJ, et al. Large scale single deletion of mitochondrial DNA in chronic progressive external ophthalmoplegia. J Korean Child Neurol Soc. 2004. 12:43–49.10. Seong MW, Hwang JM, Kim JY, Ko HS, Park SS. Molecular diagnosis for mitochondrial DNA aberrations in chronic progressive external ophthalmoplegia. J Korean Ophthalmol Soc. 2005. 46:323–329.11. Kim SH, Hwang JH, Chung KW, Kim HJ, Kim JY, Park KD, et al. Kearns-Sayre syndrome with a large deletion in mitochondrial DNA. J Korean Neurol Assoc. 2006. 24:260–264.12. Goto Y, Koga Y, Horai S, Nonaka I. Chronic progressive external ophthalmoplegia: a correlative study of mitochondrial DNA deletions and their phenotypic expression in muscle biopsies. J Neurol Sci. 1990. 100:63–69.
Article13. Kleinle S, Wiesmann U, Superti-Furga A, Krähenbühl S, Boltshauser E, Reichen J, et al. Detection and characterization of mitochondrial DNA rearrangements in Pearson and Kearns-Sayre syndromes by long PCR. Hum Genet. 1997. 100:643–650.
Article14. Stenson PD, Mort M, Ball EV, Howells K, Phillips AD, Thomas NS, et al. The Human Gene Mutation Database: 2008 update. Genome Med. 2009. 1:13.
Article15. Komaki H, Fukazawa T, Houzen H, Yoshida K, Nonaka I, Goto Y. A novel D104G mutation in the adenine nucleotide translocator 1 gene in autosomal dominant progressive external ophthalmoplegia patients with mitochondrial DNA with multiple deletions. Ann Neurol. 2002. 51:645–648.
Article16. Agostino A, Valletta L, Chinnery PF, Ferrari G, Carrara F, Taylor RW, et al. Mutations of ANT1, Twinkle, and POLG1 in sporadic progressive external ophthalmoplegia (PEO). Neurology. 2003. 60:1354–1356.
Article17. Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissière A, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy 'plus' phenotypes. Brain. 2008. 131:338–351.
Article18. Li K, Warner CK, Hodge JA, Minoshima S, Kudoh J, Fukuyama R, et al. A human muscle adenine nucleotide translocator gene has four exons, is located on chromosome 4, and is differentially expressed. J Biol Chem. 1989. 264:13998–14004.
Article19. Farge G, Pham XH, Holmlund T, Khorostov I, Falkenberg M. The accessory subunit B of DNA polymerase gamma is required for mitochondrial replisome function. Nucleic Acids Res. 2007. 35:902–911.
Article20. Korhonen JA, Gaspari M, Falkenberg M. TWINKLE Has 5'→3' DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J Biol Chem. 2003. 278:48627–48632.
Article21. Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004. 23:2423–2429.
Article22. Van Hove JL, Cunningham V, Rice C, Ringel SP, Zhang Q, Chou PC, et al. Finding twinkle in the eyes of a 71-year-old lady: a case report and review of the genotypic and phenotypic spectrum of TWINKLE-related dominant disease. Am J Med Genet A. 2009. 149A:861–867.
Article23. Virgilio R, Ronchi D, Hadjigeorgiou GM, Bordoni A, Saladino F, Moggio M, et al. Novel Twinkle (PEO1) gene mutations in mendelian progressive external ophthalmoplegia. J Neurol. 2008. 255:1384–1391.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autosomal Dominant Type of Chronic Progressive External Ophthalmoplegia With Elevated Acetylcholine Receptor Binding Antibody
- A Case of the Oculopharyngeal Muscular Dystrophy
- A Case of Chronic Progressive External Ophthalmoplegia
- Chronic progressive external ophthalmoplegia (CPEO) with 'ragged red fibers': a case report
- Molecular Diagnosis for Mitochondrial DNA Aberrations in Chronic Progressive External Ophthalmoplegia