Korean J Obstet Gynecol.
2011 Dec;54(12):770-777. 10.5468/KJOG.2011.54.12.770.
The clinicopathological relevance for the expression of secreted protein acidic and rich in cysteine in epithelial ovarian tumors
- Affiliations
-
- 1Department of Gynecology Oncology, Dongnam Institute of Radiological and Medical Sciences (DIRAMS) Cancer Center, Busan, Korea.
- 2Department of Gynecology Oncology, Pusan National University School of Medicine, Busan, Korea. msyoon@pusan.ac.kr
- KMID: 1443941
- DOI: http://doi.org/10.5468/KJOG.2011.54.12.770
Abstract
OBJECTIVE
Secreted protein acidic and rich in cysteine (SPARC) is an extracellular matrix-associated protein implicated in the modulation of cell adhesion, migration, cell cycle regulation, and angiogenesis. It has been associated with the progression or suppression of various cancers. This study was aimed at correlating SPARC protein expression with tumor progression and clinicopathological features in ovarian epithelial tumors.
METHODS
Epithelial ovarian cancer (n=69), borderline tumor (n=18), benign tumor (n=10) and normal ovary tissues were obtained after operation. SPARC protein expression was examined using immunohistochemistry. With a retrospective review, patients' characteristics and slide samples were analyzed.
RESULTS
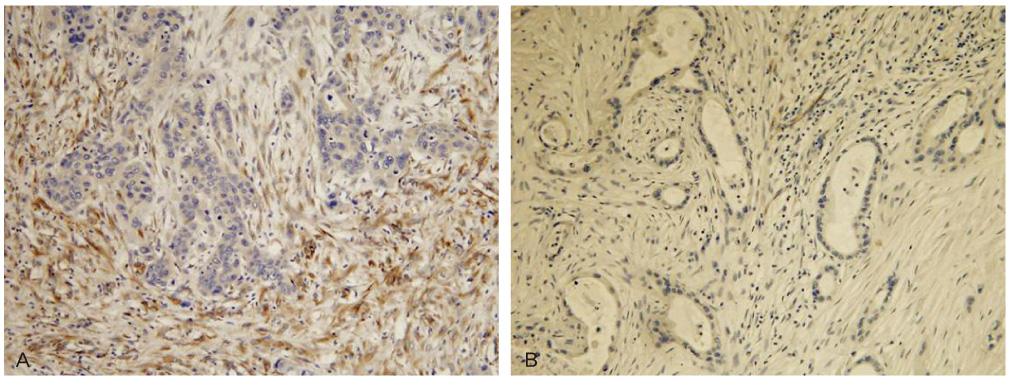
Cytoplasmic SPARC immunoreactivity was observed in stromal cells in nearly all cases of normal ovary, benign and borderline tumors (100%, 94.7%, and 100%). In contrast, SPARC was detected in the stroma of 63.8% (44/69) and the score of immunoreactivity was significantly reduced in malignant tumors (P <0.001). SPARC expression in ovarian epithelial cancers was significantly associated with International Federation of Gynecology and Obstetrics stage. However, it was not correlated with other clinicopathologic parameters, including histologic type, tumor grade, nuclear grade, mitosis, tumor size, local recurrence, distant metastasis, and survival.
CONCLUSION
This study showed that reduction of SPARC expression in ovarian epithelial tumors is significantly correlated with tumor invasiveness and SPARC may act as tumor suppressor.
MeSH Terms
Figure
Reference
-
1. Seidman JD, Russell P, Kurman RJ. Blaustein A, Kurman RJ, editors. Surface epithelial tumors of the ovary. Blaustein's pathology of the female genital tract. 2002. Vol 1:5th ed. New York: Springer-Verlag;791–810.2. Piver MS. 16,090: the 2004 estimated U.S. mortality from ovarian cancer. Gynecol Oncol. 2005. 97:301–302.3. Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005. 65:10602–10612.4. Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih Ie M. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008. 198:351–356.5. Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984. 259:3993–4007.6. Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001. 107:1049–1054.7. Francki A, Motamed K, McClure TD, Kaya M, Murri C, Blake DJ, et al. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J Cell Biochem. 2003. 88:802–811.8. Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, et al. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem. 2003. 90:408–423.9. Briggs J, Chamboredon S, Castellazzi M, Kerry JA, Bos TJ. Transcriptional upregulation of SPARC, in response to c-Jun overexpression, contributes to increased motility and invasion of MCF7 breast cancer cells. Oncogene. 2002. 21:7077–7091.10. Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, et al. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med. 1997. 3:171–176.11. Lau CP, Poon RT, Cheung ST, Yu WC, Fan ST. SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J Pathol. 2006. 210:459–468.12. Yamanaka M, Kanda K, Li NC, Fukumori T, Oka N, Kanayama HO, et al. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001. 166:2495–2499.13. Yamashita K, Upadhay S, Mimori K, Inoue H, Mori M. Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer. 2003. 97:2412–2419.14. Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002. 62:6270–6277.15. Yang E, Kang HJ, Koh KH, Rhee H, Kim NK, Kim H. Frequent inactivation of SPARC by promoter hypermethylation in colon cancers. Int J Cancer. 2007. 121:567–575.16. Mok SC, Chan WY, Wong KK, Muto MG, Berkowitz RS. SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene. 1996. 12:1895–1901.17. Mok SC, Wong KK, Chan RK, Lau CC, Tsao SW, Knapp RC, et al. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol Oncol. 1994. 52:247–252.18. Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001. 159:609–622.19. Paley PJ, Goff BA, Gown AM, Greer BE, Sage EH. Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol Oncol. 2000. 78:336–341.20. Scully RE, Young RH, Clement PB. Rosai J, editor. Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament. Atlas of tumor pathology. 1998. Washington (DC): Armed Forces Institute of Pathology;107.21. Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000. 19:7–15.22. Pecorelli S, Odicino F, Maisonneuve P, Creasman W, Shepard J, Sideri M, et al. Carcinoma of the ovary. Annual report on the results of treatment in gynaecological cancer. J Epidemiol Biostat. 1998. 3:75–102.23. Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003. 22:5021–5030.24. Nomura S, Wills AJ, Edwards DR, Heath JK, Hogan BL. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988. 106:441–450.25. Mundlos S, Schwahn B, Reichert T, Zabel B. Distribution of osteonectin mRNA and protein during human embryonic and fetal development. J Histochem Cytochem. 1992. 40:283–291.26. Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994. 8:163–173.27. Rodríguez-Jiménez FJ, Caldés T, Iniesta P, Vidart JA, Garcia-Asenjo JL, Benito M. Overexpression of SPARC protein contrasts with its transcriptional silencing by aberrant hypermethylation of SPARC CpG-rich region in endometrial carcinoma. Oncol Rep. 2007. 17:1301–1307.28. Suzuki M, Hao C, Takahashi T, Shigematsu H, Shivapurkar N, Sathyanarayana UG, et al. Aberrant methylation of SPARC in human lung cancers. Br J Cancer. 2005. 92:942–948.29. Brown TJ, Shaw PA, Karp X, Huynh MH, Begley H, Ringuette MJ. Activation of SPARC expression in reactive stroma associated with human epithelial ovarian cancer. Gynecol Oncol. 1999. 75:25–33.30. Porter PL, Sage EH, Lane TF, Funk SE, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995. 43:791–800.31. Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol. 2005. 167:1739–1752.32. Said N, Najwer I, Motamed K. Secreted protein acidic and rich in cysteine (SPARC) inhibits integrin-mediated adhesion and growth factor-dependent survival signaling in ovarian cancer. Am J Pathol. 2007. 170:1054–1063.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Secreted Protein Acidic and Rich in Cysteine Expression in Colorectal Carcinoma
- Expression of Secreted Protein Acidic and Rich in Cysteine in the Stroma of a Colorectal Carcinoma is Associated With Patient Prognosis
- Expression of p53, c-myc, Transforming Growth Factor-alpha and -beta in Human Epithelial Ovarian Tumors
- Expression of Proliferating Cell Nuclear Antigen and p53 Protein in Ovarian Epithelial Tumors
- The clinicopathological relevance of Poly (ADP-ribose) polymerase (PARP) expression in epithelial ovarian cancer