Tuberc Respir Dis.
2011 Mar;70(3):251-256. 10.4046/trd.2011.70.3.251.
A Case of Prothionamide Induced Hepatitis on Patient with Multi-Drug Resistant Pulmonary Tuberculosis
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. pms70@yuhs.ac
- 2The Institute of Chest Diseases, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1442856
- DOI: http://doi.org/10.4046/trd.2011.70.3.251
Abstract
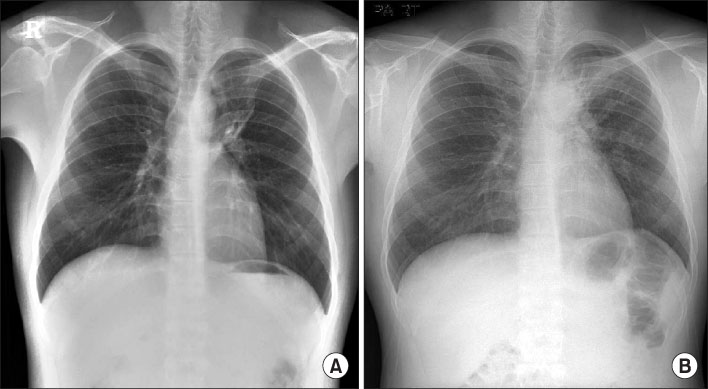
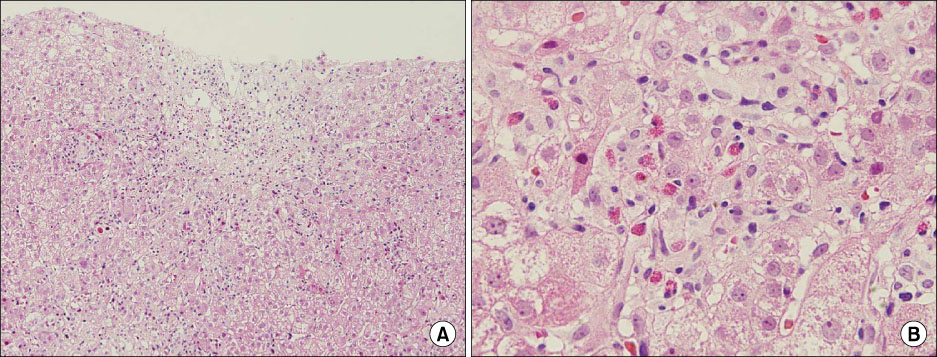
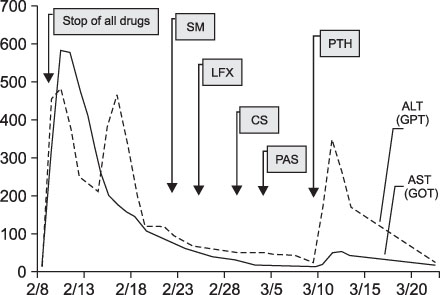
- The prevalence of multi-drug resistant tuberculosis (MDR-TB), which is resistant to isoniazid and rifampin, has been increasing in Korea. And the side effects of 2nd line anti-tuberculosis medications, including drug-induced hepatitis, are well known. Although prothionamide (PTH) is one of the most useful anti-TB medications and although TB medication-induced acute hepatitis is a severe complication, there are only a few published case reports about prothionamide induced hepatitis. In this case report, a 22 year old male was diagnosed with pulmonary MDR-TB and was administered 2nd line anti-TB mediations, including PTH. Afterwards, he had a spiking fever and his liver enzymes were more than 5 times greater than the upper limit of the normal range. He was then diagnosed with drug-induced hepatitis by liver biopsy. His symptoms and liver enzyme elevation were improved after stopping PTH. Accordingly, we report this case of an association between PTH and acute hepatitis.
MeSH Terms
Figure
Reference
-
1. The Korean Academy of Tuberculosis and Respiratory Diseases. The treatment guideline of tuberculosis. 2005. 4th ed. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases.2. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008. 23:192–202.3. Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006. 11:699–707.4. Moon DS, Jang TW, Oak CH, Jung MH, Yoo CH, Song JY, et al. A case of pyrazinamide induced fulminant hepatic failure. Tuberc Respir Dis. 2007. 63:435–439.5. McElroy PD, Ijaz K, Lambert LA, Jereb JA, Iademarco MF, Castro KG, et al. National survey to measure rates of liver injury, hospitalization, and death associated with rifampin and pyrazinamide for latent tuberculosis infection. Clin Infect Dis. 2005. 41:1125–1133.6. Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990. 11:272–276.7. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003. 167:1472–1477.8. Törün T, Güngör G, Ozmen I, Bölükbaşi Y, Maden E, Biçakçi B, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005. 9:1373–1377.9. Furin JJ, Mitnick CD, Shin SS, Bayona J, Becerra MC, Singler JM, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001. 5:648–655.10. Bloss E, Kuksa L, Holtz TH, Riekstina V, Skripconoka V, Kammerer S, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000~2004. Int J Tuberc Lung Dis. 2010. 14:275–281.11. Surucuoglu S, Ozkutuk N, Celik P, Gazi H, Dinc G, Kurutepe S, et al. Drug-resistant pulmonary tuberculosis in western Turkey: prevalence, clinical characteristics and treatment outcome. Ann Saudi Med. 2005. 25:313–318.12. Hyun JH, Kim JG, Jo SK, Yun IC. A case of toxic hepatitis induced prothionamide (TH-1321) and ethambutol. Korean J Gastroenterol. 1970. 2:21–27.13. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003. 167:603–662.14. Marzuki OA, Fauzi AR, Ayoub S, Kamarul Imran M. Prevalence and risk factors of anti-tuberculosis drug-induced hepatitis in Malaysia. Singapore Med J. 2008. 49:688–693.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical Treatment of Pulmonary Multidrug-Resistant Tuberculosis
- A clinical effect of retreatment by prothionamide, cycloserine, para-aminosalicylic acid, streptomycin(kanamycin or tuberactinomyc-in) on pulmonary tuberculosis
- A Case of Disseminated Multidrug-Resistant Tuberculosis involving the Brain
- Diagnosis and Treatment of Multidrug-Resistant Tuberculosis
- Surgical Treatment of Multidrug-resistant Pulmonary Tuberculosis