Korean J Physiol Pharmacol.
2013 Feb;17(1):1-8. 10.4196/kjpp.2013.17.1.1.
Regulation of Ca2+ Signaling in Pulmonary Hypertension
- Affiliations
-
- 1Laboratory of Genetics, Salk Institute for Biological Studies, La Jolla, California, USA. afirth@salk.edu
- 2Department of Otolaryngology, Kangwon National University Hospital, School of Medicine, Kangwon National University, Chuncheon 200-722, Korea.
- 3Department of Physiology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea. parkws@kangwon.ac.kr
- KMID: 1432756
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.1
Abstract
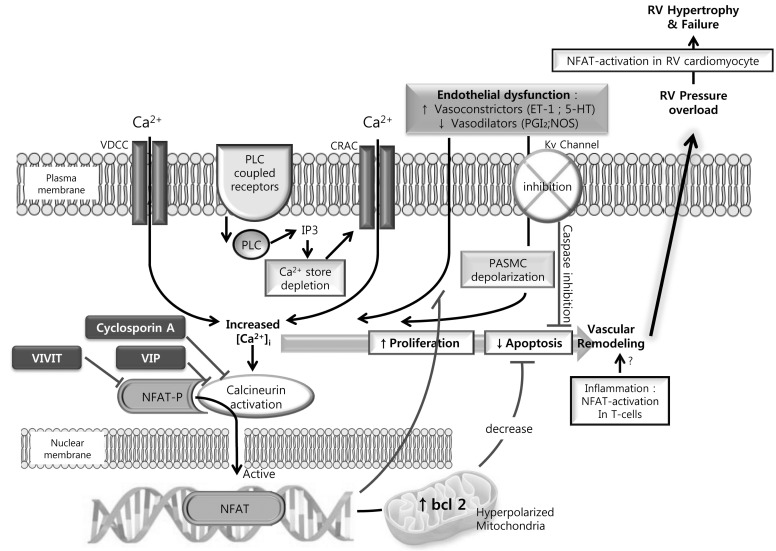
- Understanding the cellular and molecular mechanisms involved in the development and progression of pulmonary hypertension (PH) remains imperative if we are to successfully improve the quality of life and life span of patients with the disease. A whole plethora of mechanisms are associated with the development and progression of PH. Such complexity makes it difficult to isolate one particular pathway to target clinically. Changes in intracellular free calcium concentration, the most common intracellular second messenger, can have significant impact in defining the pathogenic mechanisms leading to its development and persistence. Signaling pathways leading to the elevation of [Ca2+]cyt contribute to pulmonary vasoconstriction, excessive proliferation of smooth muscle cells and ultimately pulmonary vascular remodeling. This current review serves to summarize the some of the most recent advances in the regulation of calcium during pulmonary hypertension.
MeSH Terms
Figure
Reference
-
1. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009; 54(1 Suppl):S43–S54. PMID: 19555858.
Article2. Yoon CH, Park HJ, Cho YW, Kim EJ, Lee JD, Kang KR, Han J, Kang D. Cigarette smoke extract-induced reduction in migration and contraction in normal human bronchial smooth muscle cells. Korean J Physiol Pharmacol. 2011; 15:397–403. PMID: 22359478.
Article3. Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2012; 302:H1546–H1562. PMID: 22245772.4. Wang YX, Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca2+ responses in pulmonary artery myocytes. Antioxid Redox Signal. 2010; 12:611–623. PMID: 19764882.5. Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation. 2006; 13:657–670. PMID: 17085426.6. Firth AL, Remillard CV, Platoshyn O, Fantozzi I, Ko EA, Yuan JX. Functional ion channels in human pulmonary artery smooth muscle cells: Voltage-dependent cation channels. Pulm Circ. 2011; 1:48–71. PMID: 21927714.
Article7. Platoshyn O, Yu Y, Ko EA, Remillard CV, Yuan JX. Heterogeneity of hypoxia-mediated decrease in I(K(V)) and increase in [Ca2+](cyt) in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007; 293:L402–L416. PMID: 17526598.8. Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997; 100:2347–2353. PMID: 9410914.9. Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. Hypoxic and metabolic regulation of voltage-gated K+ channels in rat pulmonary artery smooth muscle cells. Exp Physiol. 1995; 80:803–813. PMID: 8546869.10. Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995; 77:370–378. PMID: 7542182.11. Park SJ, Yoo HY, Kim HJ, Kim JK, Zhang YH, Kim SJ. Requirement of pretone by thromboxane A(2) for hypoxic pulmonary vasoconstriction in precision-cut lung slices of Rat. Korean J Physiol Pharmacol. 2012; 16:59–64. PMID: 22416221.12. Rodman DM, Harral J, Wu S, West J, Hoedt-Miller M, Reese KA, Fagan K. The low-voltage-activated calcium channel CAV3.1 controls proliferation of human pulmonary artery myocytes. Chest. 2005; 128(6 Suppl):581S–582S. PMID: 16373845.
Article13. Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res. 2005; 96:864–872. PMID: 15774856.
Article14. Pluteanu F, Cribbs LL. Regulation and function of Cav3.1 T-type calcium channels in IGF-I-stimulated pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2011; 300:C517–C525. PMID: 21148410.
Article15. Paffett ML, Riddle MA, Kanagy NL, Resta TC, Walker BR. Altered protein kinase C regulation of pulmonary endothelial store- and receptor-operated Ca2+ entry after chronic hypoxia. J Pharmacol Exp Ther. 2010; 334:753–760. PMID: 20576798.16. Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000; 525:669–680. PMID: 10856120.
Article17. Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008; 295:L104–L113. PMID: 18424621.18. Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta. 2007; 1772:895–906. PMID: 17399958.
Article19. Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001; 280:H746–H755. PMID: 11158974.20. Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L144–L155. PMID: 12060571.21. Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004; 95:496–505. PMID: 15256480.22. Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006; 98:1528–1537. PMID: 16709899.23. Lee KH. CaMKII inhibitor KN-62 blunts tumor response to hypoxia by inhibiting HIF-1α in hepatoma cells. Korean J Physiol Pharmacol. 2010; 14:331–336. PMID: 21165333.
Article24. Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA. 2004; 101:13861–13866. PMID: 15358862.
Article25. Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008; 454:538–542. PMID: 18596693.
Article26. Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008; 586:5383–5401. PMID: 18832420.
Article27. Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA. 2008; 105:2895–2900. PMID: 18287061.
Article28. Ng LC, Ramduny D, Airey JA, Singer CA, Keller PS, Shen XM, Tian H, Valencik M, Hume JR. Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2010; 299:C1079–C1090. PMID: 20739625.29. Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol. 2009; 587:2429–2442. PMID: 19332490.30. Cacoub P, Dorent R, Nataf P, Carayon A, Riquet M, Noe E, Piette JC, Godeau P, Gandjbakhch I. Endothelin-1 in the lungs of patients with pulmonary hypertension. Cardiovasc Res. 1997; 33:196–200. PMID: 9059544.31. Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol. 1994; 77:1451–1459. PMID: 7836152.
Article32. Yorikane R, Miyauchi T, Sakai S, Sakurai T, Yamaguchi I, Sugishita Y, Goto K. Altered expression of ETB-receptor mRNA in the lung of rats with pulmonary hypertension. J Cardiovasc Pharmacol. 1993; 22(Suppl 8):S336–S338. PMID: 7509980.
Article33. Liu XR, Zhang MF, Yang N, Liu Q, Wang RX, Cao YN, Yang XR, Sham JS, Lin MJ. Enhanced store-operated Ca2+ entry and TRPC channel expression in pulmonary arteries of monocrotaline-induced pulmonary hypertensive rats. Am J Physiol Cell Physiol. 2012; 302:C77–C87. PMID: 21940663.34. Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005; 115:2691–2694. PMID: 16200204.
Article35. Katayose D, Ohe M, Yamauchi K, Ogata M, Shirato K, Fujita H, Shibahara S, Takishima T. Increased expression of PDGF A- and B-chain genes in rat lungs with hypoxic pulmonary hypertension. Am J Physiol. 1993; 264:L100–L106. PMID: 8447423.
Article36. Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003; 284:C316–C330. PMID: 12529250.
Article37. Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JX. PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2012; 302:C405–C411. PMID: 22031597.38. Jin Y, Kim J, Kwak J. Activation of the cGMP/Protein kinase G pathway by nitric oxide can decrease TRPV1 activity in cultured rat dorsal root ganglion neurons. Korean J Physiol Pharmacol. 2012; 16:211–217. PMID: 22802704.
Article39. Martin E, Dahan D, Cardouat G, Gillibert-Duplantier J, Marthan R, Savineau JP, Ducret T. Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflugers Arch. 2012; 464:261–272. PMID: 22820913.
Article40. Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L555–L568. PMID: 22207590.
Article41. Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007; 104:11418–11423. PMID: 17596340.
Article42. de Frutos S, Spangler R, Alo D, Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin up-regulation. J Biol Chem. 2007; 282:15081–15089. PMID: 17403661.43. de Frutos S, Diaz JM, Nitta CH, Sherpa ML, Bosc LV. Endothelin-1 contributes to increased NFATc3 activation by chronic hypoxia in pulmonary arteries. Am J Physiol Cell Physiol. 2011; 301:C441–C450. PMID: 21525433.
Article44. Firth AL, Choi IW, Park WS. Animal models of pulmonary hypertension: Rho kinase inhibition. Prog Biophys Mol Biol. 2012; 109:67–75. PMID: 22713173.
Article45. Connolly MJ, Aaronson PI. Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther. 2011; 24:1–14. PMID: 20833255.
Article46. Yang XY, Huang CC, Kan QM, Li Y, Liu D, Zhang XC, Sato T, Yamagata S, Yamagata T. Calcium regulates caveolin-1 expression at the transcriptional level. Biochem Biophys Res Commun. 2012; 426:334–341. PMID: 22940132.
Article47. Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007; 21:2970–2979. PMID: 17470567.
Article48. Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J, Perez-Vizcaino F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res. 2006; 98:931–938. PMID: 16527989.49. Wang C, Li JF, Zhao L, Liu J, Wan J, Wang YX, Wang J, Wang C. Inhibition of SOC/Ca2+/NFAT pathway is involved in the anti-proliferative effect of sildenafil on pulmonary artery smooth muscle cells. Respir Res. 2009; 10:123. PMID: 20003325.
Article50. Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol. 2009; 47:400–410. PMID: 19540841.
Article51. Tantini B, Manes A, Fiumana E, Pignatti C, Guarnieri C, Zannoli R, Branzi A, Galie N. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol. 2005; 100:131–138. PMID: 15739122.
Article52. Kim JE, Sung JY, Woo CH, Kang YJ, Lee KY, Kim HS, Kwun WH, Choi HC. Cilostazol Inhibits Vascular Smooth Muscle Cell Proliferation and Reactive Oxygen Species Production through Activation of AMP-activated Protein Kinase Induced by Heme Oxygenase-1. Korean J Physiol Pharmacol. 2011; 15:203–210. PMID: 21994478.
Article53. Park SY, Bae JU, Hong KW, Kim CD. HO-1 Induced by Cilostazol Protects Against TNF-α-associated Cytotoxicity via a PPAR-γ-dependent Pathway in Human Endothelial Cells. Korean J Physiol Pharmacol. 2011; 15:83–88. PMID: 21660147.
Article54. Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003; 92:1162–1169. PMID: 12714563.55. Bao Y, Li R, Jiang J, Cai B, Gao J, Le K, Zhang F, Chen S, Liu P. Activation of peroxisome proliferator-activated receptor gamma inhibits endothelin-1-induced cardiac hypertrophy via the calcineurin/NFAT signaling pathway. Mol Cell Biochem. 2008; 317:189–196. PMID: 18600431.
Article56. Li Y, Connolly M, Nagaraj C, Tang B, Balint Z, Popper H, Smolle-Juettner FM, Lindenmann J, Kwapiszewska G, Aaronson PI, Wohlkoenig C, Leithner K, Olschewski H, Olschewski A. Peroxisome proliferator-activated receptor-β/δ, the acute signaling factor in prostacyclin-induced pulmonary vasodilation. Am J Respir Cell Mol Biol. 2012; 46:372–379. PMID: 22021335.
Article57. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993; 75:843–854. PMID: 8252621.
Article58. Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol. 2012; [Epub ahead of print].
Article59. Qi J, Mu D. MicroRNAs and lung cancers: from pathogenesis to clinical implications. Front Med. 2012; 6:134–155. PMID: 22528868.
Article60. Cortez MA, Welsh JW, Calin GA. Circulating microRNAs as noninvasive biomarkers in breast cancer. Recent Results Cancer Res. 2012; 195:151–161. PMID: 22527502.
Article61. Papaconstantinou I, Karakatsanis A, Gazouli M, Polymeneas G, Voros D. The role of microRNAs in liver cancer. Eur J Gastroenterol Hepatol. 2012; 24:223–228. PMID: 22228372.
Article62. Tijsen AJ, Pinto YM, Creemers EE. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012; 303:H1085–H1095. PMID: 22942181.
Article63. Li C, Pei F, Zhu X, Duan DD, Zeng C. Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction. Clin Biochem. 2012; 45:727–732. PMID: 22713968.
Article64. Thamilarasan M, Koczan D, Hecker M, Paap B, Zettl UK. MicroRNAs in multiple sclerosis and experimental autoimmune encephalomyelitis. Autoimmun Rev. 2012; 11:174–179. PMID: 21621006.
Article65. Iborra M, Bernuzzi F, Invernizzi P, Danese S. MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmun Rev. 2012; 11:305–314. PMID: 20627134.
Article66. Beveridge NJ, Cairns MJ. MicroRNA dysregulation in schizophrenia. Neurobiol Dis. 2012; 46:263–271. PMID: 22207190.
Article67. Filkova M, Jungel A, Gay RE, Gay S. MicroRNAs in rheumatoid arthritis: potential role in diagnosis and therapy. BioDrugs. 2012; 26:131–141. PMID: 22494429.68. Yang S, Banerjee S, Freitas Ad, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L521–L529. PMID: 22227207.
Article69. Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012; 185:409–419. PMID: 22161164.
Article70. Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012; 59:1006–1013. PMID: 22392900.
Article71. Bockmeyer CL, Maegel L, Janciauskiene S, Rische J, Lehmann U, Maus UA, Nickel N, Haverich A, Hoeper MM, Golpon HA, Kreipe H, Laenger F, Jonigk D. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant. 2012; 31:764–772. PMID: 22534459.
Article72. Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010; 30:716–723. PMID: 20110569.
Article73. Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010; 464:1196–1200. PMID: 20364122.
Article74. Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008; 15:272–284. PMID: 18694566.
Article75. Paulin R, Courboulin A, Barrier M, Bonnet S. From oncoproteins/tumor suppressors to microRNAs, the newest therapeutic targets for pulmonary arterial hypertension. J Mol Med (Berl). 2011; 89:1089–1101. PMID: 21761156.
Article76. Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2011; 301:H1798–H1809. PMID: 21890685.
Article77. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993; 366:575–580. PMID: 8255296.78. Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993; 75:1297–1303. PMID: 7916660.79. Janicic N, Soliman E, Pausova Z, Seldin MF, Riviere M, Szpirer J, Szpirer C, Hendy GN. Mapping of the calcium-sensing receptor gene (CASR) to human chromosome 3q13.3-21 by fluorescence in situ hybridization, and localization to rat chromosome 11 and mouse chromosome 16. Mamm Genome. 1995; 6:798–801. PMID: 8597637.
Article80. Aida K, Koishi S, Tawata M, Onaya T. Molecular cloning of a putative Ca2+-sensing receptor cDNA from human kidney. Biochem Biophys Res Commun. 1995; 214:524–529. PMID: 7677761.81. Ferry S, Traiffort E, Stinnakre J, Ruat M. Developmental and adult expression of rat calcium-sensing receptor transcripts in neurons and oligodendrocytes. Eur J Neurosci. 2000; 12:872–884. PMID: 10762317.
Article82. Vizard TN, O'Keeffe GW, Gutierrez H, Kos CH, Riccardi D, Davies AM. Regulation of axonal and dendritic growth by the extracellular calcium-sensing receptor. Nat Neurosci. 2008; 11:285–291. PMID: 18223649.
Article83. Wang Y, Awumey EK, Chatterjee PK, Somasundaram C, Bian K, Rogers KV, Dunn C, Bukoski RD. Molecular cloning and characterization of a rat sensory nerve Ca2+-sensing receptor. Am J Physiol Cell Physiol. 2003; 285:C64–C75. PMID: 12637267.84. Rasschaert J, Malaisse WJ. Expression of the calcium-sensing receptor in pancreatic islet B-cells. Biochem Biophys Res Commun. 1999; 264:615–618. PMID: 10543980.
Article85. Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone. 2010; 46:571–576. PMID: 19660583.
Article86. Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, Izumi N, Kawashima H, Ozawa H, Ikeda K, Kameda A, Hakeda Y, Kumegawa M. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun. 1998; 245:419–422. PMID: 9571166.
Article87. Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative cross-talk between calcium-sensing receptor and β-catenin signaling systems in colonic epithelium. J Biol Chem. 2012; 287:1158–1167. PMID: 22094462.
Article88. Milara J, Mata M, Serrano A, Peiro T, Morcillo EJ, Cortijo J. Extracellular calcium-sensing receptor mediates human bronchial epithelial wound repair. Biochem Pharmacol. 2010; 80:236–246. PMID: 20381461.
Article89. Xing WJ, Kong FJ, Li GW, Qiao K, Zhang WH, Zhang L, Bai SZ, Xi YH, Li HX, Tian Y, Ren H, Wu LY, Wang R, Xu CQ. Calcium-sensing receptors induce apoptosis during simulated ischaemia-reperfusion in Buffalo rat liver cells. Clin Exp Pharmacol Physiol. 2011; 38:605–612. PMID: 21692826.
Article90. Canaff L, Petit JL, Kisiel M, Watson PH, Gascon-Barre M, Hendy GN. Extracellular calcium-sensing receptor is expressed in rat hepatocytes. coupling to intracellular calcium mobilization and stimulation of bile flow. J Biol Chem. 2001; 276:4070–4079. PMID: 11071898.91. Sun J, Murphy E. Calcium-sensing receptor: a sensor and mediator of ischemic preconditioning in the heart. Am J Physiol Heart Circ Physiol. 2010; 299:H1309–H1317. PMID: 20833954.
Article92. Sun YH, Liu MN, Li H, Shi S, Zhao YJ, Wang R, Xu CQ. Calcium-sensing receptor induces rat neonatal ventricular cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006; 350:942–948. PMID: 17046714.
Article93. Hammond CM, White D, Tomic J, Shi Y, Spaner DE. Extracellular calcium sensing promotes human B-cell activation and function. Blood. 2007; 110:3985–3995. PMID: 17724142.
Article94. Guarnieri V, Valentina D'Elia A, Baorda F, Pazienza V, Benegiamo G, Stanziale P, Copetti M, Battista C, Grimaldi F, Damante G, Pellegrini F, D'Agruma L, Zelante L, Carella M, Scillitani A. CASR gene activating mutations in two families with autosomal dominant hypocalcemia. Mol Genet Metab. 2012; 107:548–552. PMID: 22789683.
Article95. Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ, Lavoie JC, Nantel SH, Song KW, Shepherd JD, Sutherland HJ, Toze CL, Davis JH, Hogge DE. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. 2003; 32:915–923. PMID: 14561993.
Article96. Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004; 35:275–282. PMID: 15200151.
Article97. Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002; 360:692–694. PMID: 12241879.
Article98. Li Y, Song YH, Rais N, Connor E, Schatz D, Muir A, Maclaren N. Autoantibodies to the extracellular domain of the calcium sensing receptor in patients with acquired hypoparathyroidism. J Clin Invest. 1996; 97:910–914. PMID: 8613543.
Article99. Manning AT, O'Brien N, Kerin MJ. Roles for the calcium sensing receptor in primary and metastatic cancer. Eur J Surg Oncol. 2006; 32:693–697. PMID: 16765016.
Article100. Peters U, Chatterjee N, Yeager M, Chanock SJ, Schoen RE, McGlynn KA, Church TR, Weissfeld JL, Schatzkin A, Hayes RB. Association of genetic variants in the calcium-sensing receptor with risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2004; 13:2181–2186. PMID: 15598778.101. Yano S, Sugimoto T, Tsukamoto T, Chihara K, Kobayashi A, Kitazawa S, Maeda S, Kitazawa R. Decrease in vitamin D receptor and calcium-sensing receptor in highly proliferative parathyroid adenomas. Eur J Endocrinol. 2003; 148:403–411. PMID: 12656660.
Article102. Chowdhury P, Pore D, Mahata N, Karmakar P, Pal A, Chakrabarti MK. Thermostable direct hemolysin down-regulates human colon carcinoma cell proliferation with the involvement of E-cadherin, and β-catenin/Tcf-4 signaling. PLoS One. 2011; 6:e20098. PMID: 21625458.
Article103. Whitfield JF. Calcium, calcium-sensing receptor and colon cancer. Cancer Lett. 2009; 275:9–16. PMID: 18725175.
Article104. Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, Zehnder D. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007; 293:F946–F955. PMID: 17537980.
Article105. Marz W, Seelhorst U, Wellnitz B, Tiran B, Obermayer-Pietsch B, Renner W, Boehm BO, Ritz E, Hoffmann MM. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J Clin Endocrinol Metab. 2007; 92:2363–2369. PMID: 17374704.106. Zhang J, Zhou J, Cai L, Lu Y, Wang T, Zhu L, Hu Q. Extracellular calcium-sensing receptor is critical in hypoxic pulmonary vasoconstriction. Antioxid Redox Signal. 2012; 17:471–484. PMID: 22098336.
Article107. Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, Fernandez RA, Zeifman A, Makino A, Dong H, Yuan JX. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res. 2012; 111:469–481. PMID: 22730443.108. Li GW, Xing WJ, Bai SZ, Hao JH, Guo J, Li HZ, Li HX, Zhang WH, Yang BF, Wu LY, Wang R, Yang GD, Xu CQ. The calcium-sensing receptor mediates hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells through MEK1/ERK1,2 and PI3K pathways. Basic Clin Pharmacol Toxicol. 2011; 108:185–193. PMID: 21073657.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of the Ca2+ signaling pathway in osteoprotegerin inhibition of osteoclast differentiation and maturation
- Role of Regulators of G-Protein Signaling 4 in Ca2+ Signaling in Mouse Pancreatic Acinar Cells
- Ca2+ signalling in endothelial cells: Role of ion channels
- H2O2 Enhances Ca2+ Release from Osteoblast Internal Stores
- Regulation of GTP-binding state in RalA through Ca2+ and calmodulin