Korean J Radiol.
2013 Feb;14(1):51-60. 10.3348/kjr.2013.14.1.51.
Detection of Recurrent Hepatocellular Carcinoma in Cirrhotic Liver after Transcatheter Arterial Chemoembolization: Value of Quantitative Color Mapping of the Arterial Enhancement Fraction of the Liver
- Affiliations
-
- 1Department of Radiology and Institute of Radiation Medicine, Seoul National University College of Medicine, Seoul 110-744, Korea. jmsh@snu.ac.kr
- 2Siemens Medical Solutions, Erlangen D-91052, Germany.
- 3Department of Radiology, National Cancer Center, Goyang 410-769, Korea.
- KMID: 1430044
- DOI: http://doi.org/10.3348/kjr.2013.14.1.51
Abstract
OBJECTIVE
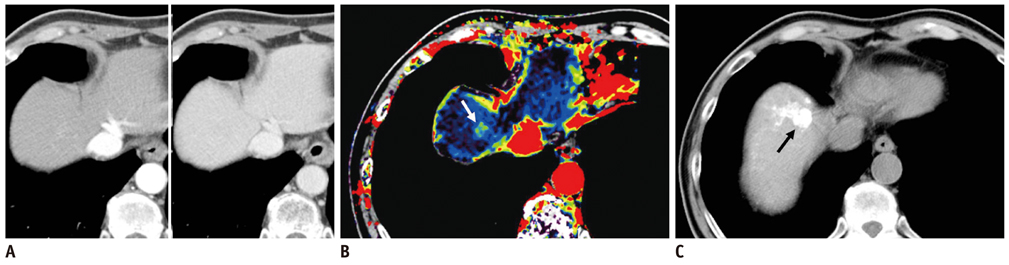
To investigate the additional diagnostic value of color mapping of the hepatic arterial enhancement fraction (AEF) for detecting recurrent or residual hepatocellular carcinoma (HCC) in patients treated with transcatheter arterial chemoembolization (TACE).
MATERIALS AND METHODS
Seventy-six patients with 126 HCCs, all of whom had undergone previous TACE, and subsequently, underwent follow-up multiphasic liver CT scans, were included in this study. Quantitative color maps of the AEF of the whole liver were created, by using prototype software with non-rigid registration. The AEF was defined as the ratio of the attenuation increment during the arterial phase to the attenuation increment during the portal phase. Two radiologists independently analyzed the two image sets at a two-week interval, i.e., the multiphasic CT image set and the second image set of the AEF color maps and the CT images. The additional diagnostic value of the AEF color mapping was determined, by the use of the jackknife-alternative free-response receiver-operating-characteristic analysis. The sensitivity and positive predictive values for detecting HCCs of each image set were also evaluated and compared.
RESULTS
The reader-averaged figures of merit were 0.699 on the initial interpretation of the MDCT image set, and 0.831 on the second interpretation of the combined image set; the difference between the two interpretations was significant (p value < 0.001). The mean sensitivity for residual or recurrent HCC detection increased from 62.7% on the initial analysis to 82.1% on the second analysis using the AEF color maps (p value < 0.001). The mean positive predictive value for HCC detection was 74.5% on the initial analysis using MDCT, and 71.6% on the second analysis using AEF color mapping.
CONCLUSION
Quantitative color mapping of the hepatic AEF may have the possibility to increase the diagnostic performance of MDCT for the detection of recurrent or residual HCC without the potential risk of radiation-related hazards.
Keyword
MeSH Terms
-
Aged
Carcinoma, Hepatocellular/pathology/*radiography
*Chemoembolization, Therapeutic
Female
Humans
Liver Cirrhosis/*complications/radiography
Liver Neoplasms/pathology/*radiography
Male
Middle Aged
Neoplasm Recurrence, Local/pathology/*radiography
Predictive Value of Tests
ROC Curve
Radiographic Image Interpretation, Computer-Assisted
Retrospective Studies
Sensitivity and Specificity
Software
*Tomography, X-Ray Computed
Figure
Reference
-
1. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983. 148:397–401.2. Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, et al. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992. 182:709–713.3. Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993. 188:79–83.4. Park JH, Han JK, Chung JW, Han MC, Kim ST. Postoperative recurrence of hepatocellular carcinoma: results of transcatheter arterial chemoembolization. Cardiovasc Intervent Radiol. 1993. 16:21–24.5. Kim SH, Lee WJ, Lim HK, Lim JH. Prediction of viable tumor in hepatocellular carcinoma treated with transcatheter arterial chemoembolization: usefulness of attenuation value measurement at quadruple-phase helical computed tomography. J Comput Assist Tomogr. 2007. 31:198–203.6. Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000. 175:699–704.7. Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008. 247:311–330.8. Kim TK, Choi BI, Han JK, Chung JW, Park JH, Han MC. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998. 208:597–603.9. Kim HC, Kim AY, Han JK, Chung JW, Lee JY, Park JH, et al. Hepatic arterial and portal venous phase helical CT in patients treated with transcatheter arterial chemoembolization for hepatocellular carcinoma: added value of unenhanced images. Radiology. 2002. 225:773–780.10. Jang KM, Choi D, Lim HK, Lim JH, Lee JY, Lee WJ, et al. Depiction of viable tumor in hepatocellular carcinoma treated with transarterial chemoembolization: multiphasic helical CT with review of the previous serial CT images. Korean J Radiol. 2005. 6:153–160.11. Kim YS, Rhim H, Lim HK, Park CK, Lee WJ, Do YS, et al. Completeness of treatment in hepatocellular carcinomas treated with image-guided tumor therapies: Evaluation of positive predictive value of contrast-enhanced CT with histopathologic correlation in the explanted liver specimen. J Comput Assist Tomogr. 2006. 30:578–582.12. Kim KW, Lee JM, Klotz E, Park HS, Lee DH, Kim JY, et al. Quantitative CT color mapping of the arterial enhancement fraction of the liver to detect hepatocellular carcinoma. Radiology. 2009. 250:425–434.13. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.14. Platt JF, Francis IR, Ellis JH, Reige KA. Difference in global hepatic enhancement assessed by dynamic CT in normal subjects and patients with hepatic metastases. J Comput Assist Tomogr. 1997. 21:348–354.15. Platt JF, Francis IR, Ellis JH, Reige KA. Liver metastases: early detection based on abnormal contrast material enhancement at dual-phase helical CT. Radiology. 1997. 205:49–53.16. Quiroga S, Sebastià C, Pallisa E, Castellà E, Pérez-Lafuente M, Alvarez-Castells A. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics. 2001. 21:65–81. questionnaire 288-294.17. White MJ, O'Gorman RL, Charles-Edwards EM, Kane PA, Karani JB, Leach MO, et al. Parametric mapping of the hepatic perfusion index with gadolinium-enhanced volumetric MRI. Br J Radiol. 2007. 80:113–120.18. Iannaccone R, Laghi A, Catalano C, Rossi P, Mangiapane F, Murakami T, et al. Hepatocellular carcinoma: role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology. 2005. 234:460–467.19. Kim YK, Kwak HS, Kim CS, Chung GH, Han YM, Lee JM. Hepatocellular carcinoma in patients with chronic liver disease: comparison of SPIO-enhanced MR imaging and 16-detector row CT. Radiology. 2006. 238:531–541.20. Chakraborty DP. Analysis of location specific observer performance data: validated extensions of the jackknife free-response (JAFROC) method. Acad Radiol. 2006. 13:1187–1193.21. Kim SH, Lee JM, Kim YJ, Choi JY, Kim GH, Lee HY, et al. Detection of hepatocellular carcinoma on CT in liver transplant candidates: comparison of PACS tile and multisynchronized stack modes. AJR Am J Roentgenol. 2007. 188:1337–1342.22. Park Y, Kim SH, Kim SH, Jeon YH, Lee J, Kim MJ, et al. Gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI versus gadobenate dimeglumine (Gd-BOPTA)-enhanced MRI for preoperatively detecting hepatocellular carcinoma: an initial experience. Korean J Radiol. 2010. 11:433–440.23. Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010. 11:295–303.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Color Doppler Ultrasound of Hepatocellular Carcinoma: Evaluation of Recurrence after Transcatheter Arterial Chemoembolization
- Palliative Transcatheter Arterial Chemoembolization for Relieving Metastatic Bone Pain due to Hepatocellular Carcinoma: A Case Report
- Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization: Difficulties on Imaging Follow-up
- Rupture of hepatocellular carcinoma after transcatheter arterial chemoembolization: A case report
- A Case of Early Multiply Recurred Hepatocellular Carcinoma after Surgical Resection in Patient Who Unprecedented Chronic Liver Disease