J Korean Med Sci.
2012 Jun;27(6):674-680. 10.3346/jkms.2012.27.6.674.
Relationship between Maternal Serum C-Reactive Protein, Funisitis and Early-Onset Neonatal Sepsis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. pkh0419@snubh.org
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 1421621
- DOI: http://doi.org/10.3346/jkms.2012.27.6.674
Abstract
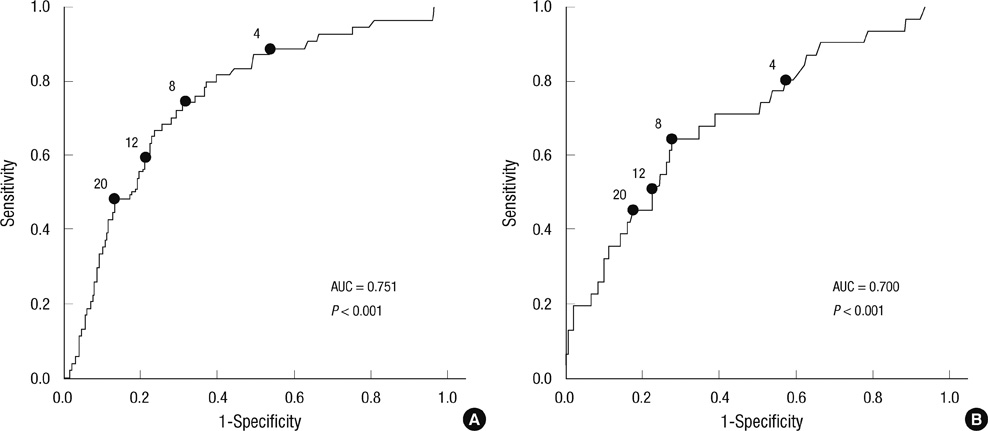
- The aim of this study was to determine whether maternal serum C-reactive protein (CRP) is of value in predicting funisitis and early-onset neonatal sepsis (EONS) in women with preterm labor or preterm premature rupture of membranes (PROM). This retrospective cohort study included 306 consecutive women with preterm labor or preterm PROM who delivered preterm singleton neonates (23-35 weeks gestation) within 72 hr of CRP measurement. The CRP level was measured with a highly sensitive immunoassay. The sensitivity, specificity, positive predictive value, and negative predictive value of an elevated serum CRP level (> or = 8 mg/L) were 74.1%, 67.5%, 32.8%, and 92.4% for funisitis, and 67.7%, 63.3%, 17.2%, and 94.6% for EONS, respectively. Logistic regression analysis demonstrated that elevated levels of serum CRP were significantly associated with funisitis and EONS, even after adjusting gestational age. The maternal serum CRP level obtained up to 72 hr before delivery is an independent predictor of funisitis and EONS in women with preterm labor or preterm PROM. A low serum CRP level (< 8 mg/L) has good negative predictive value in excluding funisitis and EONS, and may therefore be used as a non-invasive adjunct to clinical judgment to identify low-risk patients.
Keyword
MeSH Terms
-
Adult
Age of Onset
Area Under Curve
Biological Markers/blood
C-Reactive Protein/*analysis
Chorioamnionitis/blood/*diagnosis
Cohort Studies
Female
Fetal Membranes, Premature Rupture/blood
Gestational Age
Humans
Infant, Newborn
Infant, Premature
Infant, Premature, Diseases/blood/*diagnosis
*Predictive Value of Tests
Pregnancy
Premature Birth/blood
ROC Curve
Retrospective Studies
Sepsis/blood/*diagnosis
Biological Markers
C-Reactive Protein
Figure
Cited by 1 articles
-
Maternal Plasma and Amniotic Fluid LBP, Pentraxin 3, Resistin, and IGFBP-3: Biomarkers of Microbial Invasion of Amniotic Cavity and/or Intra-amniotic Inflammation in Women with Preterm Premature Rupture of Membranes
Eunwook Joo, Kyo Hoon Park, Yu Mi Kim, Kwanghee Ahn, Subeen Hong
J Korean Med Sci. 2021;36(44):e279. doi: 10.3346/jkms.2021.36.e279.
Reference
-
1. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007. 65:S194–S202.2. Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002. 8:3–13.3. Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998. 179:186–193.4. Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999. 181:773–779.5. Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000. 182:675–681.6. Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002. 11:18–25.7. Ohlsson A, Vearncombe M. Congenital and nosocomial sepsis in infants born in a regional perinatal unit: cause, outcome, and white blood cell response. Am J Obstet Gynecol. 1987. 156:407–413.8. Leviton A, Dammann O, Engelke S, Allred E, Kuban KC, O'Shea TM, Paneth N. ELGAN Study Investigators. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatr. 2010. 99:1795–1800.9. Faix RG, Donn SM. Association of septic shock caused by early-onset group B streptococcal sepsis and periventricular leukomalacia in the preterm infant. Pediatrics. 1985. 76:415–419.10. Baud O, Emilie D, Pelletier E, Lacaze-Masmonteil T, Zupan V, Fernandez H, Dehan M, Frydman R, Ville Y. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol. 1999. 106:72–77.11. Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995. 172:960–970.12. Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996. 87:231–237.13. Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, Ko EM. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001. 185:1156–1161.14. Figueroa-Damián R, Arredondo-Garcia JL, Mancilla-Ramírez J. Amniotic fluid interleukin-6 and the risk of early-onset sepsis among preterm infants. Arch Med Res. 1999. 30:198–202.15. Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, Wang E, Bhandari V. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007. 61:318–324.16. Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007. 109:121–127.17. Martius JA, Roos T, Gora B, Oehler MK, Schrod L, Papadopoulos T, Gross U. Risk factors associated with early-onset sepsis in premature infants. Eur J Obstet Gynecol Reprod Biol. 1999. 85:151–158.18. Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000. 183:1124–1129.19. Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990. 12:1179–1186.20. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982. 145:1–8.21. Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008. 121:129–134.22. Smulian JC, Bhandari V, Campbell WA, Rodis JF, Vintzileos AM. Value of umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med. 1997. 6:254–259.23. Skrablin S, Lovric H, Banovic V, Kralik S, Dijakovic A, Kalafatic D. Maternal plasma interleukin-6, interleukin-1beta and C-reactive protein as indicators of tocolysis failure and neonatal outcome after preterm delivery. J Matern Fetal Neonatal Med. 2007. 20:335–341.24. van der Heyden JL, van Teeffelen SS, Coolen AC, Halbertsma FJ, Aardenburg R, Mertens HJ, Mol BW. Is it useful to measure C-reactive protein and leukocytes in patients with prelabor rupture of membranes? Am J Perinatol. 2010. 27:543–547.25. Torbé A, Kowalski K. Maternal serum and vaginal fluid C-reactive protein levels do not predict early-onset neonatal infection in preterm premature rupture of membranes. J Perinatol. 2010. 30:655–659.26. Kurki T, Teramo K, Ylikorkala O, Paavonen J. C-reactive protein in preterm premature rupture of the membranes. Arch Gynecol Obstet. 1990. 247:31–37.27. Ernest JM, Swain M, Block SM, Nelson LH, Hatjis CG, Meis PJ. C-reactive protein: a limited test for managing patients with preterm labor or preterm rupture of membranes? Am J Obstet Gynecol. 1987. 156:449–454.28. Mazor M, Kassis A, Horowitz S, Wiznitzer A, Kuperman O, Meril C, Glezerman M. Relationship between C-reactive protein levels and intraamniotic infection in women with preterm labor. J Reprod Med. 1993. 38:799–803.29. Szarka A, Rigó J Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010. 11:59.30. Picklesimer AH, Jared HL, Moss K, Offenbacher S, Beck JD, Boggess KA. Racial differences in C-reactive protein levels during normal pregnancy. Am J Obstet Gynecol. 2008. 199:523.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Positive Maternal C-Reactive Protein Predicts Neonatal Sepsis
- Prenatal Diagnosis of Intrauterine Infection and Prediction of Neonatal Morbidity by Maternal Serum C-Reactive Protein in Patients with Preterm Premature Rupture of Membranes
- The Association between Vitamin D Levels and Neonatal Early-onset Sepsis : A Systematic Review and Meta-analysis
- Influence of histologic chorioamnionitis and funisitis on the level of peripheral blood C-reactive protein at birth in preterm infants
- Epidemiology of Early and Late Onset Neonatal Sepsis