Ann Lab Med.
2012 Jul;32(4):270-275. 10.3343/alm.2012.32.4.270.
Characterization of Carbapenemase Genes in Enterobacteriaceae Species Exhibiting Decreased Susceptibility to Carbapenems in a University Hospital in Chongqing, China
- Affiliations
-
- 1Department of Clinical Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China. xiayun12cn@yahoo.com.cn
- KMID: 1380086
- DOI: http://doi.org/10.3343/alm.2012.32.4.270
Abstract
- BACKGROUND
Our study was to investigate the prevalence of carbapenemase genes in strains of Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in our hospital.
METHODS
The carbapenemase producing Enterobacteriaceae species were confirmed by modified Hodge test (MHT) and EDTA-disc synergy test which indicating the production of class B carbapenemases. PCR and sequencing analysis were used to identify the drug-resistant genes. DNA fingerprinting based on enterobacterial repetitive intergenic consensus (ERIC)-PCR was applied to investigate the homology of Enterobacteriaceae species.
RESULTS
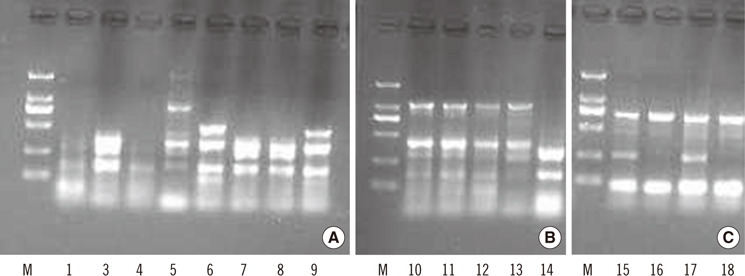
From a collection of 1,472 Enterobacteriaceae species, 18 isolates with decreased susceptibility to carbapenem treatment were identified and 9 of which were positive by MHT, and 6 of which produced class B carbapenemases. PCR and sequencing analysis of the 18 isolates revealed 4 different carbapenemase genes (blaIMP-8, blaoxa-1, blaIMP-26, and blaoxa-47) in 10 isolates, with the blaIMP-8 and blaoxa-1 genes being the most common (60-70% prevalence). ERIC-PCR showed 5, 2, and 2 unique genotypes for Enterobacter cloacae, Escherichia coli, and Klebsiella pneumoniae, respectively. Three E. coli strains isolated from different patients from the urologic surgery department exhibited the same DNA banding pattern, suggesting a possible clonal dissemination. Majority (17/18) of the carbapenem-unsusceptible Enterobacteriaceae species isolates was obtained from the surgery department of our hospital.
CONCLUSIONS
The main carbapenemase genes of Enterobacteriaceae species in our hospital were blaIMP-8 and blaoxa-1. Prevalence of carbapenem resistance may be existed in surgery department and infection control should be taken for preventing further dissemination of drug-resistant strains.
MeSH Terms
-
Anti-Bacterial Agents/*pharmacology
Bacterial Proteins/*genetics
Carbapenems/*pharmacology
China
DNA Fingerprinting
Drug Resistance, Bacterial/drug effects/genetics
Enterobacteriaceae/*drug effects/*enzymology/isolation & purification
Enterobacteriaceae Infections/microbiology
Genotype
Hospitals, University
Humans
Microbial Sensitivity Tests
Sequence Analysis, DNA
beta-Lactamases/*genetics
Figure
Reference
-
1. Oteo J, Delgado-Iribarren A, Vega D, Bautista V, Rodríguez MC, Velasco M, et al. Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int J Antimicrob Agents. 2008. 32:534–537.
Article2. Kaczmarek FM, Dib-Hajj F, Shang W, Gootz TD. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT-1) β-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin phoe. Antimicrob Agents Chemother. 2006. 50:3396–3406.3. Vatopoulos A. High rates of metallo-β-lactamase-producing Klebsiella pneumoniae in Greece--a review of the current evidence. Euro Surveill. 2008. 13:pii: 8023.4. Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007. 20:440–458.
Article5. Ambler RP, Coulson AF, Frère JM, Ghuysen JM, Joris B, Forsman M, et al. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991. 276:269–270.
Article6. Cai JC, Zhou HW, Zhang R, Chen GX. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob Agents Chemother. 2008. 52:2014–2018.7. Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007. 51:763–765.8. Walsh TR. The emergence and implications of metallo-β-lactamases in Gram-negative bacteria. Clin Microbiol Infect. 2005. 11:Suppl 6. 2–9.
Article9. Yang Q, Wang H, Sun H, Chen H, Xu Y, Chen M. Phenotypic and genotypic characterization of Enterobacteriaceae with decreased susceptibility to carbapenems: results from large hospital-based surveillance studies in China. Antimicrob Agents Chemother. 2010. 54:573–577.10. Cuzon G, Naas T, Boqaerts P, Glupczynski Y, Huang TD, Nordmann P. Plasmid-encoded carbapenem-hydrolyzing β-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob Agents Chemother. 2008. 52:3463–3464.11. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 21st Informational supplement, M100-S21. 2011. Wayne, PA: Clinical and Laboratory Standards Institute.12. Radice M, Power P, Gutkind G, Fernández K, Vay C, Famiglietti A, et al. First class a carbapenemase isolated from enterobacteriaceae in Argentina. Antimicrob Agents Chemother. 2004. 48:1068–1069.13. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum GH. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001. 7:88–91.14. Queenan AM, Torres-Viera C, Gold HS, Carmeli Y, Eliopoulos GM, Moellering RC Jr, et al. SME-type carbapenem-hydrolyzing class A β-lactamases from geographically diverse Serratia marcescens strains. Antimicrob Agents Chemother. 2000. 44:3035–3039.15. Bratu S, Tolaney P, Karumudi U, Quale J, Mooty M, Nichani S, et al. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005. 56:128–132.16. Qi C, Malczynski M, Parker M, Scheetz MH. Characterization of genetic diversity of carbapenem-resistant Acinetobacter baumannii clinical strains collected from 2004 to 2007. J Clin Microbiol. 2008. 46:1106–1109.17. Tsakris A, Pournaras S, Woodford N, Palepou MF, Babini GS, Douboyas J, et al. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol. 2000. 38:1290–1292.18. Yong D, Toleman MA, Giske CG, Cho HS, Sundman S, Lee K, et al. Characterization of a new metallo-β-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009. 53:5046–5054.19. Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004. 48:15–22.20. Manoharan A, Premalatha K, Chatterjee S, Mathai D. SARI Group. Correlation of TEM, SHV and CTX-M extended-spectrum beta lactamases among Enterobacteriaceae with their in vitro antimicrobial susceptibility. Indian J Med Microbiol. 2011. 29:161–164.21. Mammeri H, Guillon H, Eb F, Nordmann P. Phenotypic and biochemical. comparison of the carbapenem-hydrolyzing activities of five plasmid-borne AmpC β-lactamases. Antimicrob Agents Chemother. 2010. 54:4556–4560.
Article22. Smith JL, Drum DJ, Dai Y, Kim JM, Sanchez S, Maurer JJ, et al. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl Environ Microbiol. 2007. 73:1404–1414.23. Khan AA, McCarthy S, Wang RF, Cerniglia CE. Characterization of United States outbreak isolates of Vibrio parahaemolyticus using enterobacterial repetitive intergenic consensus (ERIC) PCR and development of a rapid PCR method for detection of O3:K6 isolates. FEMS Microbiol Lett. 2002. 206:209–214.24. Yan JJ, Ko WC, WU JJ. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-β-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001. 45:2368–2371.25. Yan JJ, Ko WC, Tsai SH, Wu HM, Wu JJ. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying bla(IMP-8) in a university medical center in Taiwan. J Clin Microbiol. 2001. 39:4433–4439.26. Yan JJ, Ko WC, Chuang CL, Wu JJ. Metallo-β-lactamase-producing Enterobacteriaceae isolates in a university hospital in Taiwan: prevalence of IMP-8 in Enterobacter cloacae and first identification of VIM-2 in Citrobacter freundii. J Antimicrob Chemother. 2002. 50:503–511.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emergence and Spread of OXA-48-Like Carbapenemase-Producing Enterobacteriaceae
- Evaluation of Diagnostic Performance of RAPIDEC CARBA NP Test for Carbapenemase-Producing Enterobacteriaceae
- The Infinity War: How to Cope with Carbapenem-resistant Enterobacteriaceae
- Strategies for Interpretive Standards of beta-Lactams Susceptibility Testing and Identification of Extended-Spectrum beta-Lactamases and Carbapenemases in Enterobacteriaceae
- Commentary on “Sacral Nerves Reconstruction After Surgical Resection of a Large Sacral Chordoma Restores the Urinary and Sexual Function and the Anal Continence”