J Vet Sci.
2012 Jun;13(2):187-191. 10.4142/jvs.2012.13.2.187.
Evaluation of the effect of a 0.0584% hydrocortisone aceponate spray on clinical signs and skin barrier function in dogs with atopic dermatitis
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. cyhwang@snu.ac.kr
- KMID: 1376193
- DOI: http://doi.org/10.4142/jvs.2012.13.2.187
Abstract
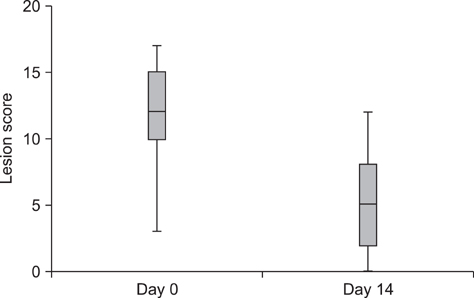
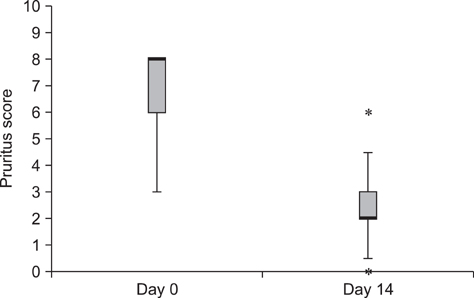
- The purpose of this study was to evaluate the effects of a topical spray containing 0.0584% hydrocortisone aceponate (HCA) on canine atopic dermatitis (CAD) and to evaluate the skin barrier function during the treatment of CAD. Twenty-one dogs that fulfilled the diagnostic criteria for CAD were included in this study. The HCA spray was applied once a day to the lesions of all dogs for 7 or 14 days. Clinical assessment was performed before (day 0) and after treatment (day 14), and clinical responses were correlated with changes in skin barrier function. CAD severity significantly decreased after 14 days of HCA treatment based on the lesion scores (p < 0.0001), which were determined using the CAD extent and severity index (CADESI-03) and pruritus scores (p < 0.0001) calculated using a pruritus visual analog scale. Transepidermal water loss, a biomarker of skin barrier function, was significantly reduced compared to baseline (day 0) measurements (p = 0.0011). HCA spray was shown to be effective for significantly improving the condition of dogs suffering from CAD. This treatment also significantly improved cutaneous hydration and skin barrier function in the animals.
MeSH Terms
Figure
Reference
-
1. Aalto-Korte K. Improvement of skin barrier function during treatment of atopic dermatitis. J Am Acad Dermatol. 1995. 33:969–972.
Article2. Behrend EN, Kemppainen RJ. Glucocorticoid therapy. Pharmacology, indications, and complications. Vet Clin North Am Small Anim Pract. 1997. 27:187–213.3. Bieber T, Vick K, Folster-Holst R, Belloni-Fortina A, Stadtler G, Worm M, Arcangeli F. Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy. 2007. 62:184–189.
Article4. Bonneau S, Skowronski V, Sanquer A, Maynard L, Eun HM. Therapeutic efficacy of topical hydrocortisone aceponate in experimental flea-allergy dermatitis in dogs. Aust Vet J. 2009. 87:287–291.
Article5. Brazzini B, Pimpinelli N. New and established topical corticosteroids in dermatology: clinical pharmacology and therapeutic use. Am J Clin Dermatol. 2002. 3:47–58.6. Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006. 118:3–21.
Article7. DeBoer DJ. Canine atopic dermatitis: new targets, new therapies. J Nutr. 2004. 134:2056S–2061S.
Article8. Elias PM. Barrier repair trumps immunology in the pathogenesis and therapy of atopic dermatitis. Drug Discov Today Dis Mech. 2008. 5:e33–e38.
Article9. Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011. 41:282–295.
Article10. Griffin CE, DeBoer DJ. The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Vet Immunol Immunopathol. 2001. 81:255–269.
Article11. Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989. 30:323–333.
Article12. Gupta AK, Chow M. Prednicarbate (Dermatop®): profile of a corticosteroid. J Cutan Med Surg. 2004. 8:244–247.
Article13. Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa'ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008. 121:725–730.
Article14. Hightower K, Marsella R, Flynn-Lurie A. Effects of age and allergen exposure on transepidermal water loss in a house dust mite-sensitized beagle model of atopic dermatitis. Vet Dermatol. 2010. 21:88–95.
Article15. Hill PB, DeBoer DJ. The ACVD task force on canine atopic dermatitis (IV): environmental allergens. Vet Immunol Immunopathol. 2001. 81:169–186.
Article16. Hillier A, Griffin CE. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet Immunol Immunopathol. 2001. 81:147–151.
Article17. Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991. 96:523–526.
Article18. Kim DW, Park JY, Na GY, Lee SJ, Lee WJ. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol. 2006. 45:698–701.
Article19. Marsella R, Samuelson D. Unravelling the skin barrier: a new paradigm for atopic dermatitis and house dust mites. Vet Dermatol. 2009. 20:533–540.
Article20. Nuttall T, Mueller R, Bensignor E, Verde M, Noli C, Schmidt V, Reme C. Efficacy of a 0.0584% hydrocortisone aceponate spray in the management of canine atopic dermatitis: a randomised, double blind, placebo-controlled trial. Vet Dermatol. 2009. 20:191–198.
Article21. Nuutinen J, Alanen E, Autio P, Lahtinen MR, Harvima I, Lahtinen T. A closed unventilated chamber for the measurement of transepidermal water loss. Skin Res Technol. 2003. 9:85–89.
Article22. Oh WS, Oh TH. Measurement of transepidermal water loss from clipped and unclipped anatomical sites on the dog. Aust Vet J. 2009. 87:409–412.
Article23. Olivry T, DeBoer DJ, Favrot C, Jackson HA, Mueller RS, Nuttall T, Prelaud P. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol. 2010. 21:233–248.
Article24. Olivry T, Hill PB. The ACVD task force on canine atopic dermatitis (VIII): is the epidermal lipid barrier defective? Vet Immunol Immunopathol. 2001. 81:215–218.
Article25. Olivry T, Mueller RS. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Dermatol. 2003. 14:121–146.
Article26. Olivry T, Mueller R, Nuttall T, Favrot C, Prelaud P. Determination of CADESI 03 thresholds for increasing severity levels of canine atopic dermatitis. Vet Dermatol. 2008. 19:115–119.
Article27. Olivry T, Sousa CA. The ACVD task force on canine atopic dermatitis (XIX): general principles of therapy. Vet Immunol Immunopathol. 2001. 81:311–316.
Article28. Olivry T, Sousa CA. The ACVD task force on canine atopic dermatitis (XX): glucocorticoid pharmacotherapy. Vet Immunol Immunopathol. 2001. 81:317–322.
Article29. Reiter LV, Torres SMF, Wertz PW. Characterization and quantification of ceramides in the nonlesional skin of canine patients with atopic dermatitis compared with controls. Vet Dermatol. 2009. 20:260–266.
Article30. Ruzicka T. Methylprednisolone aceponate in eczema and other inflammatory skin disorders -- a clinical update. Int J Clin Pract. 2006. 60:85–92.
Article31. Rybnicek J, Lau-Gillard PJ, Harvey R, Hill PB. Further validation of a pruritus severity scale for use in dogs. Vet Dermatol. 2009. 20:115–122.
Article32. Schackert C, Korting HC, Schafer-Korting M. Qualitative and quantitative assessment of the benefit-risk ratio of medium potency topical corticosteroids in vitro and in vivo: characterisation of drugs with an increased benefit-risk ratio. BioDrugs. 2000. 13:267–277.
Article33. Shimada K, Yoshihara T, Yamamoto M, Konno K, Momoi Y, Nishifuji K, Iwasaki T. Transepidermal water loss (TEWL) reflects skin barrier function of dog. J Vet Med Sci. 2008. 70:841–843.
Article34. Sugarman JL. The epidermal barrier in atopic dermatitis. Semin Cutan Med Surg. 2008. 27:108–114.
Article35. Thomas RC, Logas D, Radosta L, Harrison J. Effects of a 1% hydrocortisone conditioner on hematologic and biochemical parameters, adrenal function testing, and cutaneous reactivity to histamine in normal and pruritic dogs. Vet Ther. 2000. 1:25–34.36. Watson AL, Fray TR, Bailey J, Baker CB, Beyer SA, Markwell PJ. Dietary constituents are able to play a beneficial role in canine epidermal barrier function. Exp Dermatol. 2006. 15:74–81.
Article37. Yoshihara T, Shimada K, Momoi Y, Konno K, Iwasaki T. A new method of measuring the transepidermal water loss (TEWL) of dog skin. J Vet Med Sci. 2007. 69:289–292.
Article