J Vet Sci.
2011 Dec;12(4):379-385. 10.4142/jvs.2011.12.4.379.
Expression and regulation of Enpp2 in rat uterus during the estrous cycle
- Affiliations
-
- 1Laboratory of Veterinary Biochemistry and Molecular Biology, College of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. ebjeung@chungbuk.ac.kr
- KMID: 1365022
- DOI: http://doi.org/10.4142/jvs.2011.12.4.379
Abstract
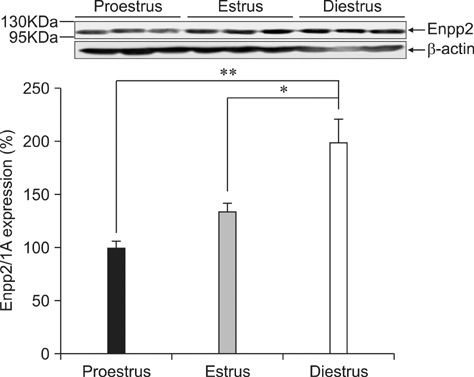
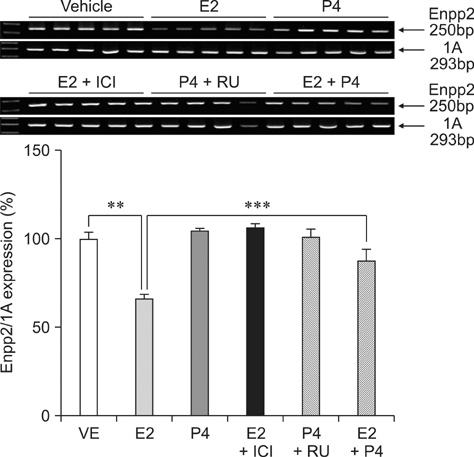
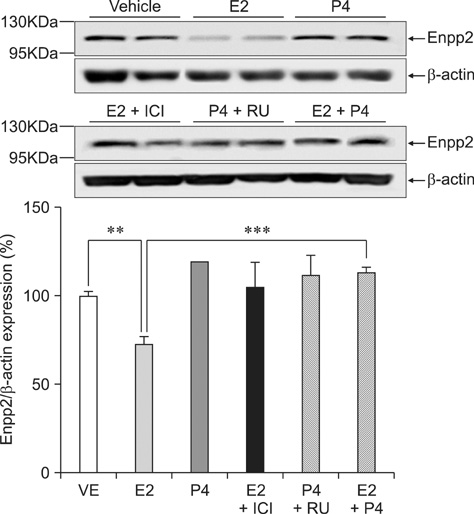
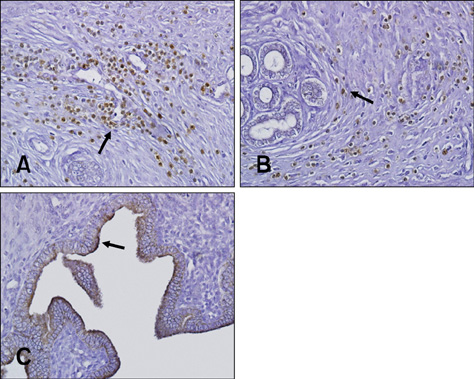
- Ectonucleotide pyrophosphatase/phosphodiestrase 2 (Enpp2) isolated from the supernatant of human melanoma cells is a lysophospholipase D that transforms lysophosphatidylcholine into lysophospatidic acid. Although multiple analyses have investigated the function of Enpp2 in the hypothalamus, its role in the uterus during the estrous cycle is not well understood. In the present study, rat uterine Enpp2 was analyzed by RT-PCR, Western blotting, and immunohistochemistry. Quantitative PCR analysis demonstrated that uterine Enpp2 mRNA was decreased during estrus compared to proestrus and diestrus. To determine whether uterine Enpp2 expression is affected by sex steroid hormones, immature rats were treated with 17beta-estradiol (E2), progesterone, or both on postnatal days 14 to 16. Interestingly, the expression of Enpp2 mRNA and protein were down-regulated by E2 in the uterus during estrus but not during proestrus or diestrus, suggesting that Enpp2 may play a role in uterine function during estrus. Enpp2 is primarily localized in the stromal cells of the endometrium during proestrus and estrus. During diestrus, Enpp2 was highly expressed in the epithelial cells of the endometrium. Taken together, these results suggest that uterine Enpp2 may be regulated by E2 and plays a role in reproductive functions during female rat development.
Keyword
MeSH Terms
Figure
Reference
-
1. Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004. 15:477–489.
Article2. Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002. 277:48737–48744.
Article3. Applezweig N. Steroids. Chem Week. 1969. 104:57–72.4. Arnold JT, Kaufman DG, Seppälä M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001. 16:836–845.
Article5. Bagchi MK, Tsai SY, Tsai MJ, O'Malley BW. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proc Natl Acad Sci USA. 1992. 89:2664–2668.
Article6. Billon-Denis E, Tanfin Z, Robin P. Role of lysophosphatidic acid in the regulation of uterine leiomyoma cell proliferation by phospholipase D and autotaxin. J Lipid Res. 2008. 49:295–307.
Article7. Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005. 19:833–842.
Article8. Boutin JA, Ferry G. Autotaxin. Cell Mol Life Sci. 2009. 66:3009–3021.
Article9. Chen SU, Lee H, Chang DY, Chou CH, Chang CY, Chao KH, Lin CW, Yang YS. Lysophosphatidic acid mediates interleukin-8 expression in human endometrial stromal cells through its receptor and nuclear factor-βB-dependent pathway: a possible role in angiogenesis of endometrium and placenta. Endocrinology. 2008. 149:5888–5896.
Article10. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987. 162:156–159.
Article11. de Greef WJ, Zeilmaker GH. Blood pregesterone levels in pseudopregnant rats: effects of partial removal of luteal tissue. Endocrinology. 1974. 95:565–571.
Article12. Fotopoulou S, Oikonomou N, Grigorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, Zhao Z, Xu Y, Kontoyiannis DL, Remboutsika E, Aidinis V. ATX expression and LPA signalling are vital for the development of the nervous system. Dev Biol. 2010. 339:451–464.
Article13. Gijsbers R, Aoki J, Arai H, Bollen M. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS Lett. 2003. 538:60–64.
Article14. Gijsbers R, Ceulemans H, Stalmans W, Bollen M. Structural and catalytic similarities between nucleotide pyrophosphatases/phosphodiesterases and alkaline phosphatases. J Biol Chem. 2001. 276:1361–1368.
Article15. Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta. 2003. 1638:1–19.
Article16. Hama K, Aoki J, Bandoh K, Inoue A, Endo T, Amano T, Suzuki H, Arai H. Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci. 2006. 79:1736–1740.
Article17. Htun H, Holth LT, Walker D, Davie JR, Hager GL. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell. 1999. 10:471–486.
Article18. Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004. 73:321–354.
Article19. Kamińska K, Wasielak M, Bogacka I, Blitek M, Bogacki M. Quantitative expression of lysophosphatidic acid receptor 3 gene in porcine endometrium during the periimplantation period and estrous cycle. Prostaglandins Other Lipid Mediat. 2008. 85:26–32.
Article20. Kehlen A, Englert N, Seifert A, Klonisch T, Dralle H, Langner J, Hoang-Vu C. Expression, regulation and function of autotaxin in thyroid carcinomas. Int J Cancer. 2004. 109:833–838.
Article21. Koh E, Clair T, Woodhouse EC, Schiffmann E, Liotta L, Stracke M. Site-directed mutations in the tumor-associated cytokine, autotaxin, eliminate nucleotide phosphodiesterase, lysophospholipase D, and motogenic activities. Cancer Res. 2003. 63:2042–2045.22. Koike S, Keino-Masu K, Ohto T, Masu M. The N-terminal hydrophobic sequence of autotaxin (ENPP2) functions as a signal peptide. Genes Cells. 2006. 11:133–142.
Article23. Lee HY, Clair T, Mulvaney PT, Woodhouse EC, Aznavoorian S, Liotta LA, Stracke ML. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J Biol Chem. 1996. 271:24408–24412.
Article24. Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Single-chain estrogen receptors (ERs) reveal that the ERα/β heterodimer emulates functions of the ERα dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004. 24:7681–7694.
Article25. Liszewska E, Reinaud P, Billon-Denis E, Dubois O, Robin P, Charpigny G. Lysophosphatidic acid signaling during embryo development in sheep: involvement in prostaglandin synthesis. Endocrinology. 2009. 150:422–434.
Article26. Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, Coombes K, Muller W, Hung MC, Perou CM, Lee AV, Fang X, Mills GB. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009. 15:539–550.
Article27. Murata J, Lee HY, Clair T, Krutzsch HC, Arestad AA, Sobel ME, Liotta LA, Stracke ML. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J Biol Chem. 1994. 269:30479–30484.
Article28. Ohkawa R, Hisano N, Nakamura K, Okubo S, Yokota H, Yatomi Y. Lysophospholipase D activity exists in the urine to catalyse the formation of lysophosphatidic acid. Nephrol Dial Transplant. 2006. 21:3612–3613.
Article29. Okudaira S, Yukiura H, Aoki J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie. 2010. 92:698–706.
Article30. Ryan KJ. Biochemistry of aromatase: significance to female reproductive physiology. Cancer Res. 1982. 42:8 Suppl. 3342s–3344s.31. Seo H, Kim M, Choi Y, Lee CK, Ka H. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology. 2008. 149:6166–6175.
Article32. Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod. 1971. 5:297–307.
Article33. Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal. 2006. 2:361–370.
Article34. Takeo C, Ikeda K, Horie-Inoue K, Inoue S. Identification of Igf2, Igfbp2 and Enpp2 as estrogen-responsive genes in rat hippocampus. Endocr J. 2009. 56:113–120.
Article35. Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006. 281:25822–25830.
Article36. Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002. 158:227–233.
Article37. van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007. 46:145–160.
Article38. Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994. 14:8356–8364.
Article39. Xie Y, Meier KE. Lysophospholipase D and its role in LPA production. Cell Signal. 2004. 16:975–981.
Article40. Ye X, Chun J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab. 2010. 21:17–24.
Article41. Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005. 435:104–108.
Article42. Yu L, Netticadan T, Xu YJ, Panagia V, Dhalla NS. Mechanisms of lysophosphatidylcholine-induced increase in intracellular calcium in rat cardiomyocytes. J Pharmacol Exp Ther. 1998. 286:1–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Luteinizing Hormone (LH) and Its Receptor Gene in Uterus from Cycling Rats
- Regulation of Estrogen Receptor mRNA in Rat Anterior Pituitary Gland
- Menstruation and Sleep
- Protein Expression of Matrix Metalloproteinases of Mouse Reproductive Organs During Estrous Cycle
- Establishment of an In Vivo Rat Model to Investigate Female Vaginal Arousal Response