Clin Orthop Surg.
2009 Mar;1(1):40-47. 10.4055/cios.2009.1.1.40.
Assessment of Bone Quality using Finite Element Analysis Based upon Micro-CT Images
- Affiliations
-
- 1Department of Internal Medicine, Endocrinology, Yonsei University, Seoul, Korea.
- 2LCT Orthropaedic Speciality Hospital, Suwon, Korea. junehh@hotmail.com
- 3Department of Orthopedic Surgery, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Mechanical Engineering, Dankook University, Yongin, Korea.
- KMID: 1127913
- DOI: http://doi.org/10.4055/cios.2009.1.1.40
Abstract
-
BACKGROUND: To evaluate the feasibility of a micro-image based finite element model to determine the efficacy of sequential treatments on the bone quality in a rat osteoporosis model.
METHODS
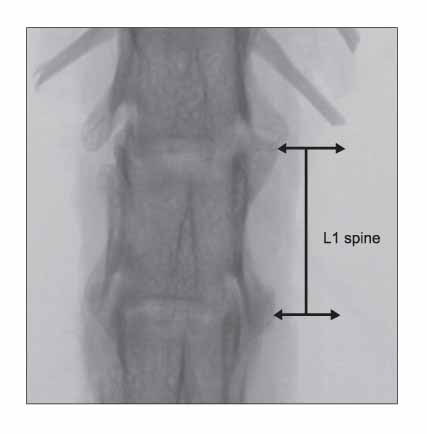
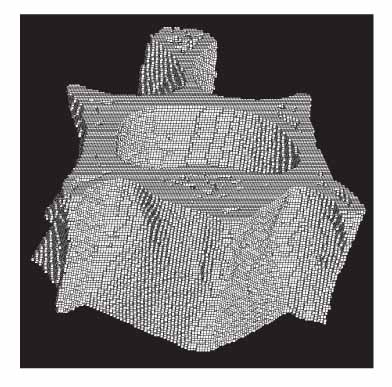
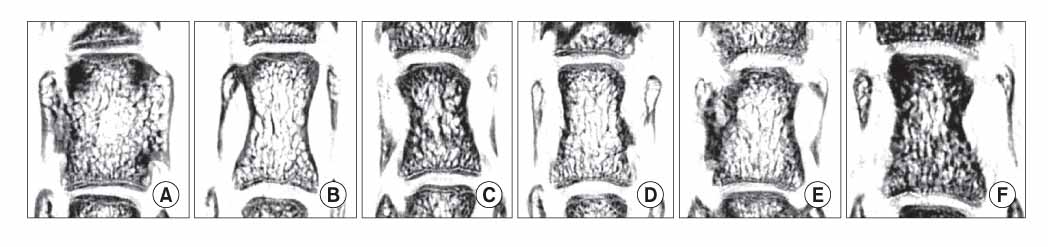
Rat osteoporosis and treated osteoporosis models were established with the bone loss, restore and maintain concept. Thirty Sprague-Dawley rats were used in this study. A sham operation or ovariectomy was performed at 20 weeks after birth, which was followed by the respective sequential trials as follows: (1) sham-operation only, (2) ovariectomy only, (3) ovariectomized rats with parathyroid hormone maintenance, (4) ovariectomized rats treated with PTH for 5 weeks and then withdrawal, (5) ovariectomized rats treated with PTH for 5 weeks and then with 17 beta-estradiol, and (6) ovariectomized rats treated with parathyroid hormone for 5 weeks and then treated with zoledronate. The histomorphometry indices were determined using the micro-images from a micro-computed tomogram. Finite element analysis was carried out to determine the mechanical properties (Stiffness and Young's modulus) of the vertebra bodies. The differences in properties between the groups were compared using ANOVA and a Bonferroni's multiple group comparison procedure.
RESULTS
The histomorphometry and mechanical properties were significantly better in groups (3) and (6) than in the groups (1) and (2) (p < 0.05). The stiffness (sigmas) and Young's modulus (E) was highest in group (3) following by group (6).
CONCLUSIONS
Finite element analysis based on micro-images provides a useful tool that reflects the changes in micro-structural and mechanical properties of a rat vertebral body with the bone loss, restore and maintain concept.
MeSH Terms
Figure
Reference
-
1. Keaveny TM, Pinilla TP, Crawford RP, Kopperdahl DL, Lou A. Systematic and random errors in compression testing of trabecular bone. J Orthop Res. 1997. 15(1):101–110.
Article2. Odgaard A, Linde F. The underestimation of Young's modulus in compressive testing of cancellous bone specimens. J Biomech. 1991. 24(8):691–698.
Article3. Zhu M, Keller TS, Spengler DM. Effects of specimen load-bearing and free surface layers on the compressive mechanical properties of cellular materials. J Biomech. 1994. 27(1):57–66.
Article4. Betancourt M, Wirfel KL, Raymond AK, Yasko AW, Lee J, Vassilopoulou-Sellin R. Osteosarcoma of bone in apatient with primary hyperparathyroidism: a case report. J Bone Miner Res. 2003. 18(1):163–166.
Article5. Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002. 30(3):312–321.
Article6. Sato M, Zeng GQ, Turner CH. Biosynthetic human parathyroid hormone (1-34) effects on bone quality in aged ovariectomized rats. Endocrinology. 1997. 138(10):4330–4337.
Article7. Iwaniec UT, Samnegård E, Cullen DM, Kimmel DB. Maintenance of cancellous bone in ovariectomized, human parathyroid hormone [hPTH(1-84)]-treated rats by estrogen, risedronate, or reduced hPTH. Bone. 2001. 29(4):352–360.
Article8. Wronski TJ, Yen CF, Qi H, Dann LM. Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology. 1993. 132(2):823–831.
Article9. Green JR. Chemical and biological prerequisites for novel bisphosphonate molecules: results of comparative preclinical studies. Semin Oncol. 2001. 28(2 Suppl 6):4–10.
Article10. Rüegsegger P, Koller B, Müller R. A microtomographic system for the nondestructive evaluation of bone archi-tecture. Calcif Tissue Int. 1996. 58(1):24–29.
Article11. Muller R, Rüegsegger P. Micro-tomographic imaging for the nondestructive evaluation of trabecular bone architecture. Stud Health Technol Inform. 1997. 40:61–79.12. Ulrich D, van Rietbergen B, Weinans H, Rüegsegger P. Finite element analysis of trabecular bone structure: a comparison of image-based meshing techniques. J Biomech. 1998. 31(12):1187–1192.
Article13. van Rietbergen B, Weinans H, Huiskes R, Odgaard A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech. 1995. 28(1):69–81.
Article14. National Institutes of Health. Osteoporosis prevention, diag-nosis, and therapy. NIH Consens Statement. 2000. 17(1):1–45.15. Keaveny TM, Borchers RE, Gibson LJ, Hayes WC. Theoretical analysis of the experimental artifact in trabecular bone compressive modulus. J Biomech. 1993. 26(4-5):599–607.
Article16. Keaveny TM, Borchers RE, Gibson LJ, Hayes WC. Trabecular bone modulus and strength can depend on specimen geometry. J Biomech. 1993. 26(8):991–1000.
Article17. Ladd AJ, Kinney JH, Haupt DL, Goldstein SA. Finite-element modeling of trabecular bone: comparison with mechanical testing and determination of tissue modulus. J Orthop Res. 1998. 16(5):622–628.
Article18. Linde F, Hvid I, Madsen F. The effect of specimen geometry on the mechanical behaviour of trabecular bone specimens. J Biomech. 1992. 25(4):359–368.
Article19. Dempster WT, Liddicoat RT. Compact bone as a non-isotropic material. Am J Anat. 1952. 91(3):331–362.
Article20. Evans FG. Mechanical properties of bone. 1973. Illinois: Charles C Thomas, Springfield.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the Microstructural and Mechanical Properties in the Medial Condyle of Human Distal Femur in Advanced Osteoarthritis
- Analysis on correlation between bone strength by FEA, micro-CT parameters and bone mineral density
- Analysis of Trabecular Bone Strength using Finite Element Analysis
- Stress distribution following face mask application using different finite element models according to Hounsfield unit values in CT images
- Influence of microthread design on marginal cortical bone strain developement: A finite element analysis