Yonsei Med J.
2010 Mar;51(2):212-218. 10.3349/ymj.2010.51.2.212.
Day-to-Day Differences in Cortisol Levels and Molar Cortisol-to-DHEA Ratios among Working Individuals
- Affiliations
-
- 1Department of Statistics, Chonnam National University, Gwangju, Korea.
- 2CHA Biomedical Center, CHA Medical University, Seoul, Korea. ryunsup@yahoo.co.kr
- KMID: 1126020
- DOI: http://doi.org/10.3349/ymj.2010.51.2.212
Abstract
- PURPOSE
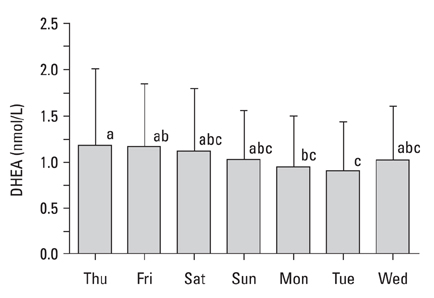
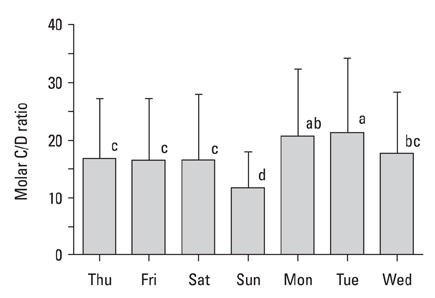
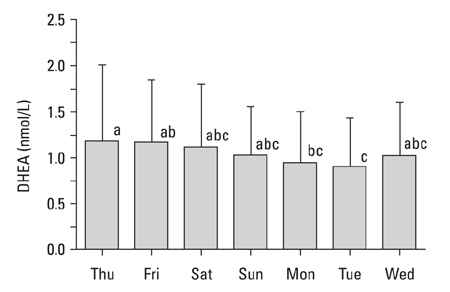
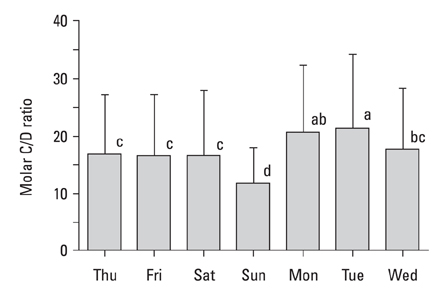
The present study was carried out to determine day-to-day differences in cortisol levels and the molar cortisol-to-dehydroepiandrosterone (DHEA) ratio (molar C/D ratio) in working subjects. MATERIALS AND METHODS: The cortisol and DHEA levels were measured from saliva samples collected 30 minutes after awakening for 7 consecutive days in full-time working subjects that worked Monday through Saturday. To determine the day-to-day differences within subjects, the collected data was analyzed using variance (ANOVA) for a randomized complete block design (RCBD). RESULTS: The cortisol levels from samples collected 30 minutes after awakening on workdays were similar to each other, but were significantly different from the cortisol levels on Sunday. The DHEA levels were not significantly different between the days of week. The DHEA levels on Monday and Tuesday were relatively lower than the levels on the other weekdays. The DHEA levels on Thursday and Friday were relatively higher than the other days. The molar C/D ratios on Sunday were significantly lower than those on workdays. The molar C/D ratios on Monday and Tuesday were significantly higher than those on Wednesday or other workdays. CONCLUSION: The cortisol levels and the molar C/D ratios demonstrate differences in adrenocortical activities between workdays and non-workdays, but the molar C/D ratio additionally represents differences in adrenocortical status between the first two workdays and other workdays. Thus, it is possible that the day-to-day differences in the cortisol levels and the molar C/D ratio represent the adrenal response to upcoming work-related stress.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cortisol Awakening Response and Nighttime Salivary Cortisol Levels in Healthy Working Korean Subjects
Il-young Shin, Ryun-sup Ahn, Sae-il Chun, Young-jin Lee, Min-soo Kim, Chea-kwan Lee, Simon Sung
Yonsei Med J. 2011;52(3):435-444. doi: 10.3349/ymj.2011.52.3.435.
Reference
-
1. Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001. 26:613–622.
Article2. Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997. 61:2539–2549.
Article3. Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise Health. 2000. 2:79–88.4. Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007. 32:358–366.5. Federenko I, Wüst S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004. 29:174–184.
Article6. Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004. 7:29–37.7. Soucy P, Luu-The V. Conversion of pregnenolone to DHEA by human 17 alpha-hydroxylase/17,20-lyase (P450c17). Evidence that DHEA is produced from the released intermediate, 17 alpha-hydroxypregnenolone. Eur J Biochem. 2000. 267:3243–3247.
Article8. Blauer KL, Poth M, Rogers WM, Bernton EW. Dehydroepiandrosterone antagonizes the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology. 1991. 129:3174–3179.9. May M, Holmes E, Rogers W, Poth M. Protection from glucocorticoid induced thymic involution by dehydroepiandrosterone. Life Sci. 1990. 46:1627–1631.10. McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002. 23:921–939.11. Goodyer IM, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: a community-based longitudinal enquiry. Psychol Med. 2003. 33:601–610.12. Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry. 2002. 159:1237–1239.
Article13. Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. Eur Neuropsychopharmacol. 2004. 14:267–273.
Article14. van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001. 26:591–612.15. Pollard TM, Ungpakorn G, Harrison GA, Parkes KR. Epinephrine and cortisol responses to work: a test of the models of Frankenhaeuser and Karasek. Ann Behav Med. 1996. 18:229–237.16. Vidovic M, Hisheh S, Schmitt LH. Cortisol and testosterone levels on a weekend and a work day in three mountain villages in the Selska Valley of northwest Slovenia. Ann Hum Biol. 2007. 34:26–33.17. Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004. 29:516–528.
Article18. Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med. 2004. 66:207–214.19. Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006. 31:1009–1018.20. Maina G, Palmas A, Filon FL. Relationship between self-reported mental stressors at the workplace and salivary cortisol. Int Arch Occup Environ Health. 2008. 81:391–400.21. Croft GP, Walker AE. Are the Monday Blues All in the Mind? The role of expectancy in the subjective experience of mood. J Appl Soc Psychol. 2001. 31:1133–1145.22. Larsen RJ, Kasimatis M. Individual differences in entrainment of mood to the weekly calendar. J Pers Soc Psychol. 1990. 58:164–171.
Article23. Willich SN, Löwel H, Lewis M, Hörmann A, Arntz HR, Keil U. Weekly variation of acute myocardial infarction. Increased Monday risk in the working population. Circulation. 1994. 90:87–93.24. Kinjo K, Sato H, Sato H, Shiotani I, Kurotobi T, Ohnishi Y, et al. Variation during the week in the incidence of acute myocardial infarction: increased risk for Japanese women on Saturdays. Heart. 2003. 89:398–403.
Article25. Helzer JE, Badger GJ, Searles JS, Rose GL, Mongeon JA. Stress and alcohol consumption in heavily drinking men: 2 years of daily data using interactive voice response. Alcohol Clin Exp Res. 2006. 30:802–811.26. Ahn RS, Lee YJ, Choi JY, Kwon HB, Chun SI. Salivary cortisol and DHEA levels in the Korean population: age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei Med J. 2007. 48:379–388.27. Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001. 26:165–173.
Article28. Corrie JE, Ratcliffe WA, Macpherson JS. Generally applicable 125 iodine-based radioimmunoassays for plasma progesterone. Steroids. 1981. 38:709–717.
Article29. Box GEP, Hunter WG, Hunter JS. Statistics for experiments-An introduction to design, data analysis and model building. 1978. New York: John Wiley & Sons;208–218.30. Kirschbaum C, Prüssner JC, Stone AA, Federenko I, Gaab J, Lintz D, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995. 57:468–474.
Article31. Levine S. Ursin H, Baade E, Levine S, editors. Cortisol changes following repeated experiences with parachute training. The psychobiology of Stress-A Study of Coping Men. 1978. New York: Academic Press;51–56.
Article32. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003. 43:2–15.
Article33. Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom Med. 1968. 30:Suppl. 576–607.
Article34. Areni CS, Burger M. Memories of "Bad" days are more biased than memories of "Good" days: past saturdays vary, but past mondays are always blue. J Appl Soc Psychol. 2008. 38:1395–1415.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exploring Subjective Stress, Sleep and Diurnal Variation of Salivary Cortisol in Korean Female Adults
- An Exploratory Study on Occupational Stress and Anxiety Through Salivary Cortisol and Self-Report Scale in Korean Nurses on Shift and Regular Work
- Salivary Cortisol and DHEA Levels in the Korean Population: Age-Related Differences, Diurnal Rhythm, and Correlations with Serum Levels
- Neurosteroid Levels in Patients with Obsessive-Compulsive Disorder
- Changes in Plasma Dehydroepiandrosterone-Sulfate ( DHEA-S ) Level & DHEA-S / cortisol Ratio by Age in Healthy Korean