Yonsei Med J.
2007 Jun;48(3):379-388. 10.3349/ymj.2007.48.3.379.
Salivary Cortisol and DHEA Levels in the Korean Population: Age-Related Differences, Diurnal Rhythm, and Correlations with Serum Levels
- Affiliations
-
- 1Graduate School of Complementary and Alternative Medicine, PoChon CHA Medical University, Seoul, Korea. chunscam@yahoo.com
- 2Hormone Research Center, Chonnam National University, Kwangju, Korea.
- KMID: 724091
- DOI: http://doi.org/10.3349/ymj.2007.48.3.379
Abstract
- PURPOSE
The primary objective of this study was to examine the changes of basal cortisol and DHEA levels present in saliva and serum with age, and to determine the correlation coefficients of steroid concentrations between saliva and serum. The secondary objective was to obtain a standard diurnal rhythm of salivary cortisol and DHEA in the Korean population.
MATERIALS AND METHODS
For the first objective, saliva and blood samples were collected between 10 and 11 AM from 359 volunteers ranging from 21 to 69 years old (167 men and 192 women). For the second objective, four saliva samples (post-awakening, 11AM, 4PM, and bedtime) were collected throughout a day from 78 volunteers (42 women and 36 men) ranging from 20 to 40 years old. Cortisol and DHEA levels were measured using a radioimmunoassay (RIA).
RESULTS
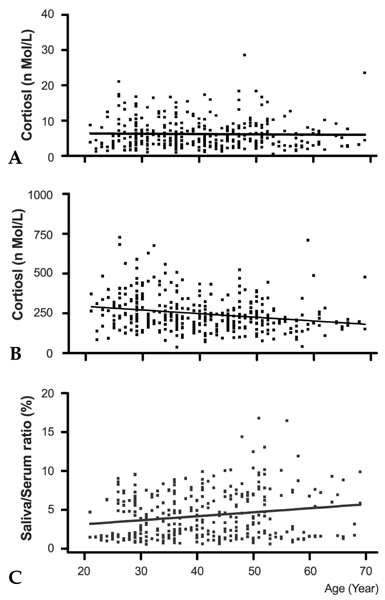
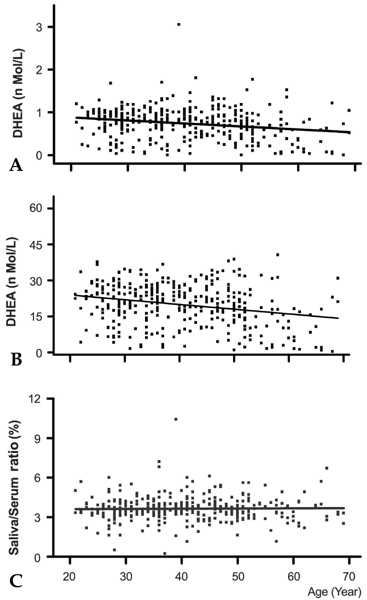
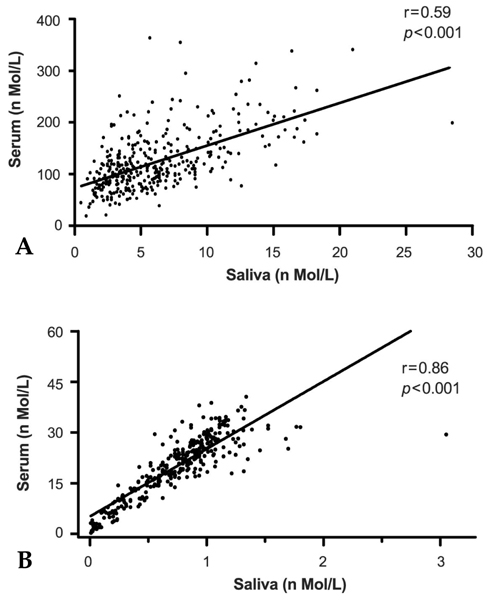
The morning cortisol and DHEA levels, and the age-related steroid decline patterns were similar in both genders. Serum cortisol levels significantly decreased around forty years of age (p < 0.001, when compared with people in their 20s), and linear regression analysis with age showed a significant declining pattern (slope= -2.29, t= -4.297, p < 0.001). However, salivary cortisol levels did not change significantly with age, but showed a tendency towards decline (slope= -0.0078, t= -0.389, p=0.697). The relative cortisol ratio of serum to saliva was 3.4 - 4.5% and the ratio increased with age (slope=0.051, t=3.61, p < 0.001). DHEA levels also declined with age in saliva (slope= -0.007, t= -3.76, p < 0.001) and serum (slope= -0.197 t= -4.88, p < 0.001). In particular, DHEA levels in saliva and serum did not start to significantly decrease until ages in the 40s, but then decreased significantly further at ages in the 50s (p < 0.001, when compared with the 40s age group) and 60s (p < 0.001, when compared with the 50 age group). The relative DHEA ratio of serum to saliva was similar throughout the ages examined (slop = 0.0016, t = 0.344, p = 0.73). On the other hand, cortisol and DHEA levels in saliva reflected well those in serum (r = 0.59 and 0.86, respectively, p < 0.001). The highest salivary cortisol levels appeared just after awakening (about two fold higher than the 11 AM level), decreased throughout the day, and reached the lowest levels at bedtime (p < 0.001, when compared with PM cortisol levels). The highest salivary DHEA levels also appeared after awakening (about 1.5 fold higher than the 11 AM level) and decreased by 11AM (p < 0.001). DHEA levels did not decrease further until bedtime (p=0.11, when compared with PM DHEA levels).
CONCLUSION
This study showed that cortisol and DHEA levels change with age and that the negative slope of DHEA was steeper than that of cortisol in saliva and serum. As the cortisol and DHEA levels in saliva reflected those in serum, the measurement of steroid levels in saliva provide a useful and practical tool to evaluate adrenal functions, which are essential for clinical diagnosis.
MeSH Terms
Figure
Cited by 1 articles
-
Cortisol Awakening Response and Nighttime Salivary Cortisol Levels in Healthy Working Korean Subjects
Il-young Shin, Ryun-sup Ahn, Sae-il Chun, Young-jin Lee, Min-soo Kim, Chea-kwan Lee, Simon Sung
Yonsei Med J. 2011;52(3):435-444. doi: 10.3349/ymj.2011.52.3.435.
Reference
-
1. DeGroot LJ, Jameson JL. Endocrinology. 2005. 5th ed. Philadelphia: Elsevier Saunders;2287–2297.2. Johnson KL, Rn CR. The hypothalamic-pituitary-adrenal axis in critical illness. AACN Clin Issues. 2006. 17:39–49.
Article3. Jefferies WM. Cortisol and immunity. Med Hypotheses. 1991. 34:198–208.
Article4. Schleimer RP. Interactions between the hypothalamic- pituitary-adrenal axis and allergic inflammation. J Allergy Clin Immunol. 2000. 106(5 Suppl):270–274.5. Rosmond R, Dallman MF, Bjornatorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998. 83:1853–1859.
Article6. de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003. 37:51–68.7. Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol. 1995. 132:705–711.
Article8. Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol. 1995. 268:R865–R873.
Article9. Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994. 14:2893–2903.
Article10. Ferrari E, Magri F, Dori D, Migliorati G, Nescis T, Molla G, et al. Neuroendocrine correlates of the aging brain in humans. Neuroendocrinology. 1995. 61:464–470.
Article11. Maes M, Calabrese J, Lee M, Meltzer HY. Effects of age on spontaneous cortisolaemia of normal volunteers and depressed patients. Psychoneuroendocrinology. 1994. 19:79–84.
Article12. Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 1985. 61:439–443.
Article13. Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005. 90:3106–3114.
Article14. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996. 81:2468–2473.
Article15. Zhao ZY, Xie Y, Fu YR, Li YY, Bogdan A, Touitou Y. Circadian rhythm characteristics of serum cortisol and dehydroepiandrosterone sulfate in healthy Chinese men aged 30 to 60 years. A cross-sectional study. Steroids. 2003. 68:133–138.
Article16. Castro M, Elias PC, Martinelli CE Jr, Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000. 33:1171–1175.
Article17. Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983. 20:329–335.
Article18. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994. 19:313–333.
Article19. Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004. 7:29–37.
Article20. Trilck M, Flitsch J, Ludecke DK, Jung R, Petersenn S. Salivary cortisol measurement-a reliable method for the diagnosis of Cushing's syndrome. Exp Clin Endocrinol Diabetes. 2005. 113:225–230.
Article21. Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: a simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology. 1999. 24:567–579.
Article22. Groschl M, Wagner R, Rauh M, Dorr HG. Stability of salivary steroids: the influences of storage, food and dental care. Steroids. 2001. 66:737–741.
Article23. Nahoul K, Patricot MC, Bressot N, Penes MC, Revol A. Measurement of salivary cortisol with four commercial kits. Ann Biol Clin (Paris). 1996. 54:75–82.24. Kwon HB, Ahn RS. Relative roles of theca and granulosa cells in ovarian follicular steroidogenesis in the amphibian, Rana nigromaculata. Gen Comp Endocrinol. 1994. 94:207–214.
Article25. Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, et al. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol. 2004. 24:2593–2604.
Article26. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle- aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002. 87:589–598.
Article27. MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin Endocrinol (Oxf). 1992. 36:339–345.
Article28. Burger HG. The endocrinology of the menopause. J Steroid Biochem Mol Biol. 1999. 69:31–35.29. Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002. 40:1151–1160.
Article30. Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003. 149:583–589.
Article31. Lukas WD, Campbell BC, Ellison PT. Testosterone, aging, and body composition in men from Harare, Zimbabwe. Am J Hum Biol. 2004. 16:704–712.
Article32. Robertson DM, Burger HG. Reproductive hormones: ageing and the perimenopause. Acta Obstet Gynecol Scand. 2002. 81:612–616.
Article33. Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997. 82:2396–2402.
Article34. Alesci S, Koch CA, Bornstein SR, Pacak K. Adrenal androgens regulation and adrenopause. Endocr Regul. 2001. 35:95–100.35. Valenti G. Adrenopause: an imbalance between dehydroepiandrosterone (DHEA) and cortisol secretion. J Endocrinol Invest. 2002. 25(10 Suppl):29–35.36. Rehman KS, Carr BR. Sex differences in adrenal androgens. Semin Reprod Med. 2004. 22:349–360.
Article37. Parker CR Jr, Mixon RL, Brissie RM, Grizzle WE. Aging alters zonation in the adrenal cortex of men. J Clin Endocrinol Metab. 1997. 82:3898–3901.
Article38. Staton BA, Mixon RL, Dharia S, Brissie RM, Parker CR Jr. Is reduced cell size the mechanism for shrinkage of the adrenal zona reticularis in aging? Endocr Res. 2004. 30:529–534.
Article39. Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf). 2000. 53:739–747.
Article40. Wedekind D, Bandelow B, Broocks A, Hajak G, Ruther E. Salivary, total plasma and plasma free cortisol in panic disorder. J Neural Transm. 2000. 107:831–837.
Article41. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989. 22:150–169.
Article42. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004. 29:83–98.
Article43. Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. 2005. 87:299–304.
Article44. Castro M, Elias PC, Martinelli CE Jr, Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000. 33:1171–1175.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Day-to-Day Differences in Cortisol Levels and Molar Cortisol-to-DHEA Ratios among Working Individuals
- Exploring Subjective Stress, Sleep and Diurnal Variation of Salivary Cortisol in Korean Female Adults
- Comparison of salivary and serum cortisol levels in mechanically ventilated patients and non-critically ill patients
- Neurosteroid Levels in Patients with Obsessive-Compulsive Disorder
- An Exploratory Study on Occupational Stress and Anxiety Through Salivary Cortisol and Self-Report Scale in Korean Nurses on Shift and Regular Work