Korean J Radiol.
2007 Aug;8(4):302-310. 10.3348/kjr.2007.8.4.302.
A New and Simple Practical Plane Dividing Hepatic Segment 2 and 3 of the Liver: Evaluation of Its Validity
- Affiliations
-
- 1Department of Radiology and Institute of Radiation Medicine, Seoul National University College of Medicine, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. chungjw@radcom.snu.ac.kr
- KMID: 1110727
- DOI: http://doi.org/10.3348/kjr.2007.8.4.302
Abstract
OBJECTIVE
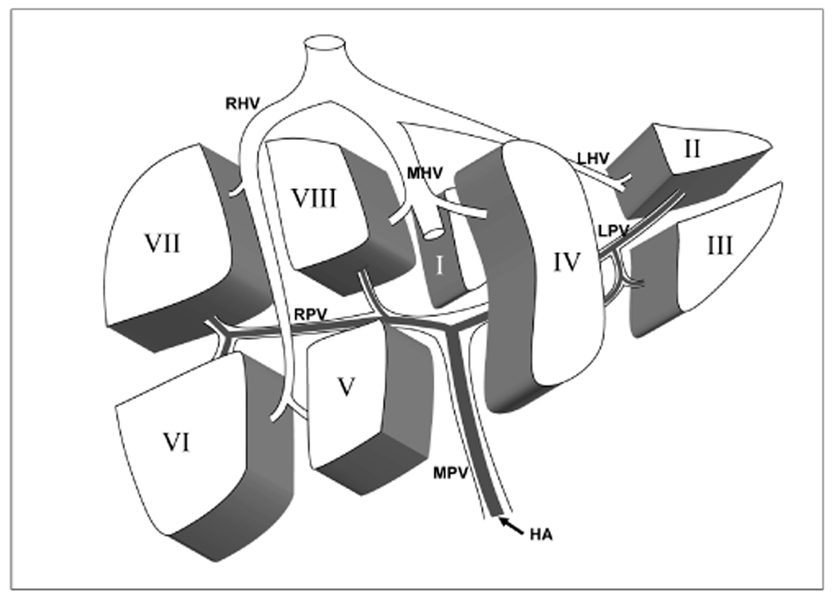
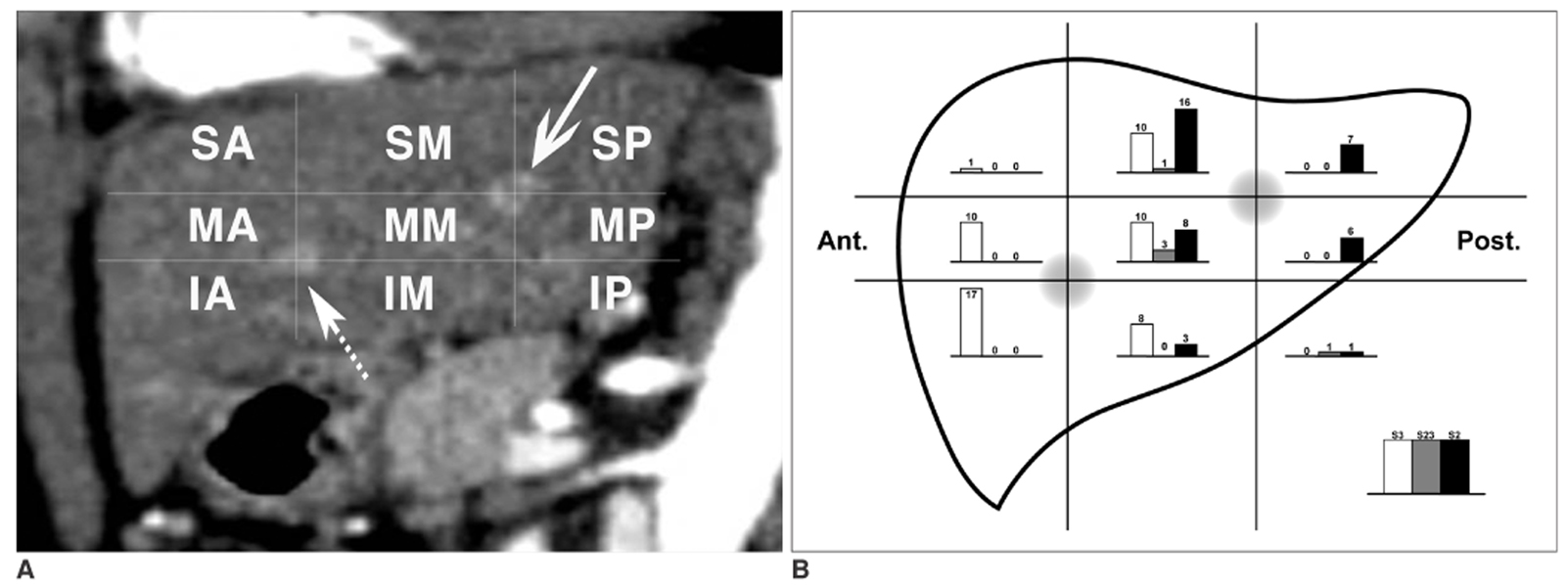
The conventional method of dividing hepatic segment 2 (S2) and 3 (S3) is subjective and CT interpretation is unclear. The purpose of our study was to test the validity of our hypothesis that the actual plane dividing S2 and S3 is a vertical plane of equal distance from the S2 and S3 portal veins in clinical situations. MATERIALS AND METHODS: We prospectively performed thin-section iodized-oil CT immediately after segmental chemoembolization of S2 or S3 in 27 consecutive patients and measured the angle of intersegmental plane on sagittal multiplanar reformation (MPR) images to verify its vertical nature. Our hypothetical plane dividing S2 and S3 is vertical and equidistant from the S2 and S3 portal veins (vertical method). To clinically validate this, we retrospectively collected 102 patients with small solitary hepatocellular carcinomas (HCC) on S2 or S3 the segmental location of which was confirmed angiographically. Two reviewers predicted the segmental location of each tumor at CT using the vertical method independently in blind trials. The agreement between CT interpretation and angiographic results was analyzed with Kappa values. We also compared the vertical method with the horizontal one. RESULTS: In MPR images, the average angle of the intersegmental plane was slanted 15 degrees anteriorly from the vertical plane. In predicting the segmental location of small HCC with the vertical method, the Kappa value between CT interpretation and angiographic result was 0.838 for reviewer 1 and 0.756 for reviewer 2. Inter-observer agreement was 0.918. The vertical method was superior to the horizontal method for localization of HCC in the left lobe (p < 0.0001 for reviewers 1 and 2). CONCLUSION: The proposed vertical plane equidistant from S2 and S3 portal vein is simple to use and useful for dividing S2 and S3 of the liver.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Angiography, Digital Subtraction
Antibiotics, Antineoplastic/administration & dosage
Carcinoma, Hepatocellular/blood supply/radiography/therapy
Chemoembolization, Therapeutic
Contrast Media
Doxorubicin/administration & dosage
Female
Humans
Iodized Oil/diagnostic use
Liver/*blood supply/*radiography
Liver Neoplasms/blood supply/radiography/therapy
Male
Middle Aged
Prospective Studies
Registries
Retrospective Studies
*Tomography, Spiral Computed
Figure
Reference
-
1. Couinaud C. Surgical Anatomy of the Liver. 1989. Revised. Paris, France: Personal.2. Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1992. 6:3–9.3. Soyer P. Segmental anatomy of the liver: utility of a nomenclature accepted worldwide. AJR Am J Roentgenol. 1993. 161:572–573.4. Nelson RC, Chezmar JL, Sugarbaker PH, Murray DR, Bernardino ME. Preoperative localization of focal liver lesions to specific liver segments: utility of CT during arterial portography. Radiology. 1990. 176:89–94.5. Lafortune M, Madore E, Patriquin H, Breton G. Segmental anatomy of the liver: a sonographic approach to the Couinaud nomenclature. Radiology. 1991. 181:443–448.6. Ferrucci JT. Liver tumor imaging: current concepts. AJR Am J Roentgenol. 1990. 155:473–484.7. Russell E, Yrizzary JM, Montalvo BM, Guerra JJ Jr, al-Refai F. Left hepatic duct anatomy: implications. Radiology. 1990. 174:353–356.8. Soyer P, Roche A, Gad M, Shapeero L, Breittmayer F, Elias D, et al. Preoperative segmental localization of hepatic metastases: utility of three-dimensional CT during arterial portography. Radiology. 1991. 180:653–658.9. Fischer L, Cardenas C, Thorn M, Benner A, Grenacher L, Vetter M, et al. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. J Comput Assist Tomogr. 2002. 26:962–967.10. Fasel JH, Selle D, Evertsz CJ, Terrier F, Peitgen HO, Gailloud P. Segmental anatomy of the liver: poor correlation with CT. Radiology. 1998. 206:151–156.11. Gupta SC, Gupta CD, Arora AK. Intrahepatic branching patterns of portal vein. A study by corrosion cast. Gastroenterology. 1977. 72:621–624.12. Lamade W, Glombitza G, Fischer L, Chiu P, Cardenas CE, Thorn M, et al. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000. 135:1256–1261.13. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003. 228:303–308.14. Ohashi I, Ina H, Okada Y, Yoshida T, Gomi N, Himeno Y, et al. Segmental anatomy of the liver under the right diaphragmatic dome: evaluation with axial CT. Radiology. 1996. 200:779–783.15. Downey PR. Radiologic identification of liver segments. AJR Am J Roentgenol. 1994. 163:1267–1268.16. International Anatomical Nomenclature Committee (IANC). Nomina anatomica. 1983. 5th ed. Baltimore, MD: Williams and Wilkins;A37.17. Bennett WF, Bova JG, Petty L, Martin EW Jr. Preoperative 3D rendering of MR imaging in liver metastases. J Comput Assist Tomogr. 1991. 15:979–984.18. van Leeuwen MS, Fernandez MA, van Es HW, Stokking R, Dillon EH, Feldberg MA. Variations in venous and segmental anatomy of the liver: two- and three-dimensional MR imaging in healthy volunteers. AJR Am J Roentgenol. 1994. 162:1337–1345.19. Deshpande RR, Heaton ND, Rela M. Surgical anatomy of segmental liver transplantation. Br J Surg. 2002. 89:1078–1088.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of liver scanning image in various hepatic disease
- Portal Venous Anatomy in Right Lobe of the Liver: CT Evaluation
- Analysis of Anatomic Variation of the Hepatic Artery by Spiral CT Hepatic Arteriography, Lipiodol CT, and Angiography
- Variations in the origin of middle hepatic artery: a cadaveric study and implications for living donor liver transplantation
- A case of simple type Caroli's disease confined to one segment of the liver