J Korean Med Sci.
2008 Dec;23(6):1039-1045. 10.3346/jkms.2008.23.6.1039.
Expression of NAD(P)H Oxidase Subunits and Their Contribution to Cardiovascular Damage in Aldosterone/Salt-Induced Hypertensive Rat
- Affiliations
-
- 1Samsung Biomedical Research Institutue, Seoul, Korea.
- 2Ottawa Health Research Institute, University of Ottawa, Ottawa, Canada.
- 3Department of Medicine/Cardiology, Cheil General Hospital, Kwandong University College of Medicine, Seoul, Korea. parkjb@skku.edu
- KMID: 1107531
- DOI: http://doi.org/10.3346/jkms.2008.23.6.1039
Abstract
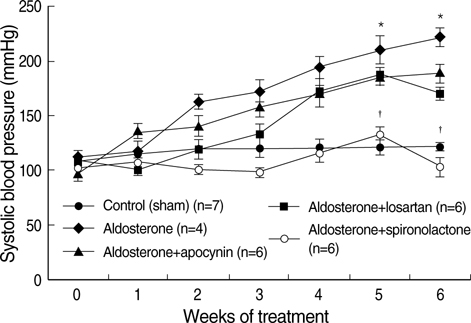
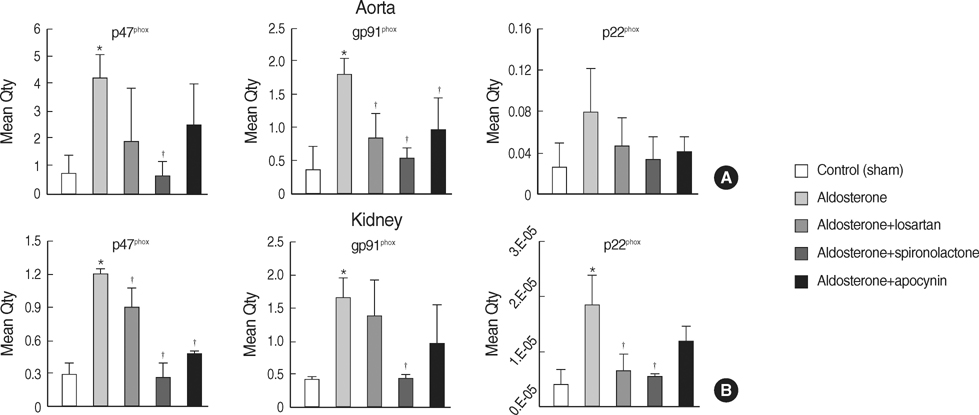
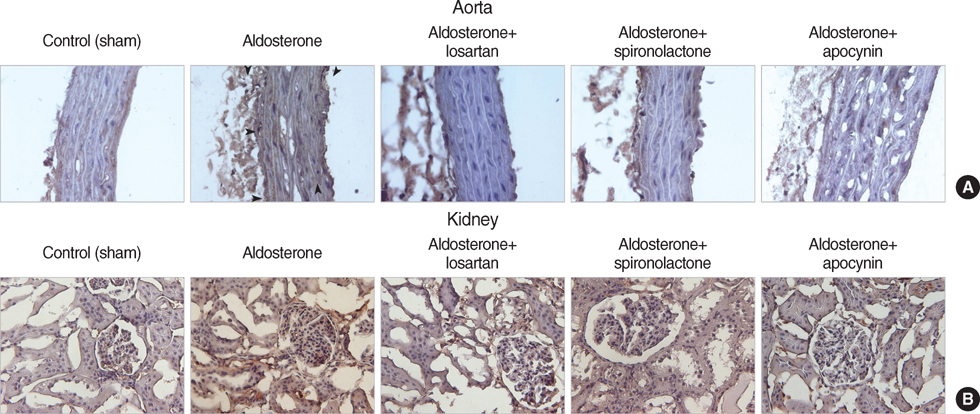
- NAD(P)H oxidase plays an important role in hypertension and its complication in aldosterone-salt rat. We questioned whether NAD(P)H oxidase subunit expression and activity are modulated by aldosterone and whether this is associated with target- organ damage. Rats were infused with aldosterone (0.75 microgram/hr/day) for 6 weeks and were given 0.9% NaCl+/-losartan (30 mg/kg/day), spironolactone (200 mg/kg/ day), and apocynin (1.5 mM/L). Aldosterone-salt hypertension was prevented completely by spironolactone and modestly by losartan and apocynin. Aldosterone increased aortic NAD(P)H oxidase activity by 34% and spironolactone and losartan inhibited the activity. Aortic expression of the subunits p47(phox), gp91(phox), and p22(phox) increased in aldosterone-infused rats by 5.5, 4.7, and 3.2-fold, respectively, which was decreased completely by spironolactone and partially by losartan and apocynin. Therefore, the increased expression of NAD(P)H oxidase may contribute to cardiovascular damage in aldosterone-salt hypertension through the increased expression of each subunit.
Keyword
MeSH Terms
-
Acetophenones/administration & dosage
Aldosterone/administration & dosage/*toxicity
Aldosterone Antagonists/administration & dosage
Angiotensin II Type 1 Receptor Blockers/administration & dosage
Animals
Anti-Inflammatory Agents, Non-Steroidal/administration & dosage
Aorta/metabolism/pathology
Blood Pressure/drug effects
Hypertension/chemically induced/drug therapy/*enzymology
Kidney/metabolism/pathology
Losartan/administration & dosage
Male
NADPH Oxidase/antagonists & inhibitors/*metabolism
Organ Size
Oxidative Stress
Protein Subunits/metabolism
RNA, Messenger/metabolism
Rats
Rats, Sprague-Dawley
Sodium Chloride/administration & dosage
Spironolactone/administration & dosage
Superoxides/metabolism
Figure
Reference
-
1. Park JB, Schiffrin EL. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens. 2002. 15:164–169.
Article2. Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004. 109:2213–2220.3. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999. 341:709–717.4. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003. 348:1309–1321.
Article5. Losel R, Schultz A, Boldyreff B, Wehling M. Rapid effects of aldosterone on vascular cells: clinical implications. Steroids. 2004. 69:575–578.
Article6. Oberleithner H. Aldosterone makes human endothelium stiff and vulnerable. Kidney Int. 2005. 67:1680–1682.
Article7. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002. 161:1773–1781.8. Gerling IC, Sun Y, Ahokas RA, Wodi LA, Bhattacharya SK, Warrington KJ, Postlethwaite AE, Weber KT. Aldosteronism: an immunostimulatory state precedes proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol. 2003. 285:H813–H821.
Article9. Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun. 2004. 313:812–817.
Article10. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004. 43:841–848.
Article11. Calò LA, Zaghetto F, Pagnin E, Davis PA, De Mozzi P, Sartorato P, Martire G, Fiore C, Armanini D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab. 2004. 89:1973–1976.12. Park JB, Schiffrin EL. ET(A) receptor antagonist prevents blood pressure elevation and vascular remodeling in aldosterone-infused rats. Hypertension. 2001. 37:1444–1449.13. Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Etayo JC, Díez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000. 35:1055–1061.
Article14. Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001. 38:606–611.
Article15. Park JB, Touyz RM, Chen X, Schiffrin EL. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2002. 15:78–84.
Article16. Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000. 101:1722–1728.
Article17. Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996. 271:23317–23321.18. Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q 4th, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997. 80:45–51.
Article19. Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001. 38:1107–1111.
Article20. Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000. 91:21–27.
Article21. Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004. 37:1542–1549.
Article22. Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005. 288:H37–H42.
Article23. Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001. 104:79–84.
Article24. Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest. 2001. 108:1513–1522.
Article25. Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002. 90:143–150.26. Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005. 28:925–936.
Article27. Lee YS, Kim JA, Kim KL, Jang HS, Kim JM, Lee JY, Shin IS, Lee JS, Suh W, Choi JH, Jeon ES, Byun J, Kim DK. Aldosterone upregulates connective tissue growth factor gene expression via p38 MAPK pathway and mineralocorticoid receptor in ventricular myocytes. J Korean Med Sci. 2004. 19:805–811.
Article28. Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005. 111:420–427.
Article29. Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens. 2005. 18:910–916.
Article30. Yoshrida K, Kim-Mitsuyama S, Wake R, Izumiya Y, Izumi Y, Yukimura T, Ueda M, Yoshiyama M, Iwao H. Excess aldosterone under normal salt diet induces cardiac hypertrophy and infiltration via oxidative stress. Hypertens Res. 2005. 28:447–455.
Article31. Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003. 42:811–817.32. Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond). 2006. 110:243–253.
Article33. Struthers AD, MacDonald TM. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res. 2004. 61:663–670.
Article34. Sorooshian M, Olson JL, Meyer TW. Effect of angiotensin II blockade on renal injury in mineralocorticoid-salt hypertension. Hypertension. 2000. 36:569–574.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of NAD (P) H Oxidase in a Potential Link between Diabetes and Vascular Smooth Muscle Cell Proliferation
- Salt, Hypertension, and Cardiovascular Diseases
- Involvement of Vascular NAD(P)H Oxidase-derived Superoxide in Cerebral Vasospasm after Subarachnoid Hemorrhage in Rats
- High Glucose and/or Free Fatty Acid Damage Vascular Endothelial Cells via Stimulating of NAD(P)H Oxidase-induced Superoxide Production from Neutrophils
- 15-Deoxy-Delta(12,14)-Prostaglandin J2 Upregulates the Expression of LPS-Induced IL-8/CXCL8 mRNA in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats