Korean J Radiol.
2009 Dec;10(6):596-603. 10.3348/kjr.2009.10.6.596.
The Antitumor Effect and Hepatotoxicity of a Hexokinase II Inhibitor 3-Bromopyruvate: In Vivo Investigation of Intraarterial Administration in a Rabbit VX2 Hepatoma Model
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Institute of Radiation Medicine, Seoul National University Medical Research Center and Clinical Research Institute, Seoul National University Hospital, Korea. chungjw@radcom.snu.ac.kr
- 2Department of Radiology, Konkuk University School of Medicine, Konkuk University Hospital, Seoul 143-729, Korea.
- 3Department of Veterinary Radiology, College of Veterinary Medicine, Chonbuk National University, Chonbuk 561-756, Korea.
- 4Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Korea.
- 5Biomedical Research Center, Korea Institute of Science and Technology, Seoul 139-774, Korea.
- KMID: 1102563
- DOI: http://doi.org/10.3348/kjr.2009.10.6.596
Abstract
OBJECTIVE
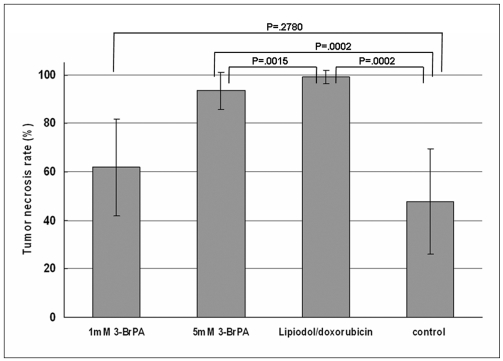
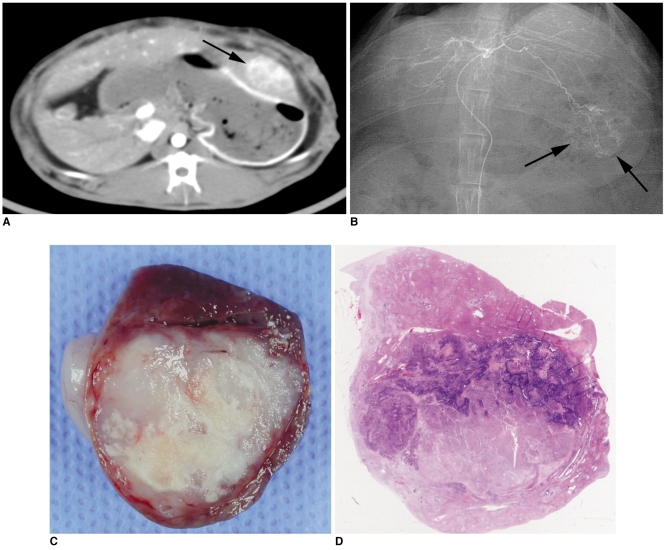
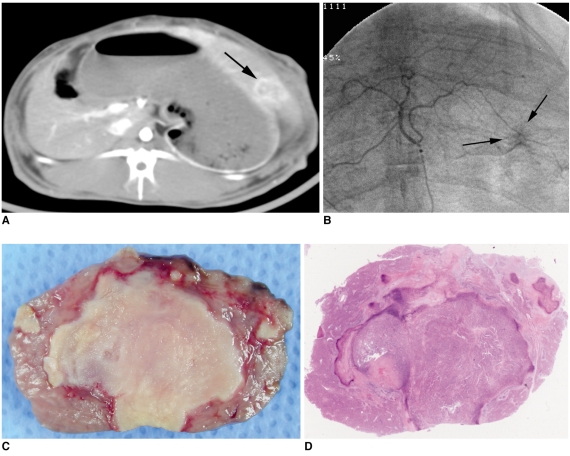
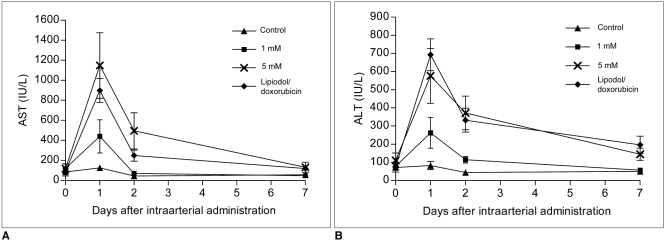
The purpose of this study was to compare the antitumor effect and hepatotoxicity of an intraarterial delivery of low-dose and high-dose 3-bromopyruvate (3-BrPA) and those of a conventional Lipiodol-doxorubicin emulsion in a rabbit VX2 hepatoma model. MATERIALS AND METHODS: This experiment was approved by the animal care committee at our institution. VX2 carcinoma was implanted in the livers of 36 rabbits. Transcatheter intraarterial administration was performed using low dose 3-BrPA (25 mL in a 1 mM concentration, n = 10), high dose 3-BrPA (25 mL in a 5 mM concentration, n = 10) and Lipiodol-doxorubicin emulsion (1.6 mg doxorubicin/ 0.4 mL Lipiodol, n = 10), and six rabbits were treated with normal saline alone as a control group. One week later, the proportion of tumor necrosis was calculated based on histopathologic examination. The hepatotoxicity was evaluated by biochemical analysis. The differences between these groups were statistically assessed with using Mann-Whitney U tests and Kruskal-Wallis tests. RESULTS: The tumor necrosis rate was significantly higher in the high dose group (93% +/- 7.6 [mean +/- SD]) than that in the control group (48% +/- 21.7) (p = 0.0002), but the tumor necrosis rate was not significantly higher in the low dose group (62% +/- 20.0) (p = 0.2780). However, the tumor necrosis rate of the high dose group was significantly lower than that of the Lipiodol-doxorubicin treatment group (99% +/- 2.7) (p = 0.0015). The hepatotoxicity observed in the 3-BrPA groups was comparable to that of the Lipiodol-doxorubicin group. CONCLUSION: Even though intraarterial delivery of 3-BrPA shows a dose-related antitumor effect, single session treatment seems to have limited efficacy when compared with the conventional method.
Keyword
MeSH Terms
-
Animals
Disease Models, Animal
Dose-Response Relationship, Drug
Doxorubicin/administration & dosage/pharmacology
Infusions, Intra-Arterial
Iodized Oil/administration & dosage/pharmacology
Liver Neoplasms, Experimental/*drug therapy/radiography
Pyruvates/administration & dosage/*pharmacology
Rabbits
Statistics, Nonparametric
Tomography, X-Ray Computed
Figure
Reference
-
1. Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999; 19:271–285. PMID: 10518307.
Article2. Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992; 15:948–963. PMID: 1314774.
Article3. Bismuth H, Houssin D, Ornowski J, Meriggi F. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986; 10:311–317. PMID: 3010585.
Article4. Bruix J, Cirera I, Calvet X, Fuster J, Bru C, Ayuso C, et al. Surgical resection and survival in Western patients with hepatocellular carcinoma. J Hepatol. 1992; 15:350–355. PMID: 1332998.
Article5. Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996; 77:2217–2222. PMID: 8635087.
Article6. Bruix J. Treatment of hepatocellular carcinoma. Hepatology. 1997; 25:259–262. PMID: 9021931.
Article7. Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972; 32:2007–2016. PMID: 4343003.8. Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978; 22:190–274. PMID: 149996.
Article9. Bustamante E, Morris HP, Pedersen PL. Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J Biol Chem. 1981; 256:8699–8704. PMID: 7263678.
Article10. Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999; 24:68–72. PMID: 10098401.
Article11. Rempel A, Mathupala SP, Griffin CA, Hawkins AL, Pedersen PL. Glucose catabolism in cancer cells: amplification of the gene encoding type II hexokinase. Cancer Res. 1996; 56:2468–2471. PMID: 8653677.12. Smith TA. Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci. 2000; 57:170–178. PMID: 10912295.13. Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells. Isolation, sequence, and activity of the promoter for type II hexokinase. J Biol Chem. 1995; 270:16918–16925. PMID: 7622509.14. Johansson T, Berrez JM, Nelson BD. Evidence that transcription of the hexokinase gene is increased in a rapidly growing rat hepatoma. Biochem Biophys Res Commun. 1985; 133:608–613. PMID: 4084289.
Article15. Rempel A, Bannasch P, Mayer D. Differences in expression and intracellular distribution of hexokinase isoenzymes in rat liver cells of different transformation stages. Biochim Biophys Acta. 1994; 1219:660–668. PMID: 7948023.
Article16. Shinohara Y, Ichihara J, Terada H. Remarkably enhanced expression of the type II hexokinase in rat hepatoma cell line AH130. FEBS Lett. 1991; 291:55–57. PMID: 1936251.
Article17. Viitanen PV, Geiger PJ, Erickson-Viitanen S, Bessman SP. Evidence for functional hexokinase compartmentation in rat skeletal muscle mitochondria. J Biol Chem. 1984; 259:9679–9686. PMID: 6746664.
Article18. Pedersen PL. Mitochondrial events in the life and death of animal cells: a brief overview. J Bioenerg Biomembr. 1999; 31:291–304. PMID: 10665520.19. Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004; 324:269–275. PMID: 15465013.
Article20. Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001; 173:83–91. PMID: 11578813.
Article21. Ko YH, McFadden BA. Alkylation of isocitrate lyase from Escherichia coli by 3-bromopyruvate. Arch Biochem Biophys. 1990; 278:373–380. PMID: 2183722.
Article22. Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002; 62:3909–3913. PMID: 12124317.23. Shin SW, Han H, Choo SW, Yoo BC, Park CK, Do YS, et al. Hepatic intra-arterial injection of 3-bromopyruvate in rabbit VX2 tumor. Acta Radiol. 2006; 47:1036–1041. PMID: 17135005.
Article24. Vali M, Liapi E, Kowalski J, Hong K, Khwaja A, Torbenson MS, et al. Intraarterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol. 2007; 18:95–101. PMID: 17296709.
Article25. Watanabe D, Ueo H, Inoue H, Matsuoka H, Honda M, Shinomiya Y, et al. Antitumor effects of intraarterial infusion of tumor necrosis factor/lipiodol emulsion on hepatic tumor in rabbits. Oncology. 1995; 52:76–81. PMID: 7800348.
Article26. Okada M, Kudo S, Miyazaki O, Saino T, Ekimoto H, Iguchi H, et al. Antitumoral efficacy and pharmacokinetic properties of pirarubicin upon hepatic intra-arterial injection in the rabbit VX2 tumour model. Br J Cancer. 1995; 71:518–524. PMID: 7880733.27. Park HS, Chung JW, Jae HJ, Kim YI, Son KR, Lee MJ, et al. FDG-PET for evaluating the antitumor effect of intraarterial 3-bromopyruvate administration in a rabbit VX2 liver tumor model. Korean J Radiol. 2007; 8:216–224. PMID: 17554189.
Article28. Yoon CJ, Chung JW, Park JH, Yoon YH, Lee JW, Jeong SY, et al. Transcatheter arterial chemoembolization with paclitaxel-lipiodol solution in rabbit VX2 liver tumor. Radiology. 2003; 229:126–131. PMID: 12944599.
Article29. Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005; 42:358–364. PMID: 15710218.
Article30. Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta. 2002; 1555:14–20. PMID: 12206885.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- FDG-PET for Evaluating the Antitumor Effect of Intraarterial 3-Bromopyruvate Administration in a Rabbit VX2 Liver Tumor Model
- Development of Rabbit Brain Tumor Model Using VX2 Cells and Verification with the MRI in Neuroradiologic Research
- Effect of Thalidomide on the Angiogenesis and In vivo Growth of Hepatoma Cells in the Mouse
- Establishment and evaluation of the VX2 orthotopic lung cancer rabbit model: a ultra-minimal invasive percutaneous puncture inoculation method
- Evaluation of Angiogenesis of Hepatic VX2 Carcinoma and Abscess in the Rabbit Liver with Perfusion Computed Tomography