Korean J Radiol.
2008 Apr;9(2):119-127. 10.3348/kjr.2008.9.2.119.
Advanced Gastric Cancer and Perfusion Imaging Using a Multidetector Row Computed Tomography: Correlation with Prognostic Determinants
- Affiliations
-
- 1Department of Radiology, Ruijin Hospital, affiliated to Jiaotong University, Shanghai, China.
- 2Department of Surgery, Ruijin Hospital, affiliated to Jiaotong University, Shanghai, China. huanzhangy@yahoo.com, yaoweiwuhuan@yahoo.com.cn
- KMID: 1098190
- DOI: http://doi.org/10.3348/kjr.2008.9.2.119
Abstract
OBJECTIVE
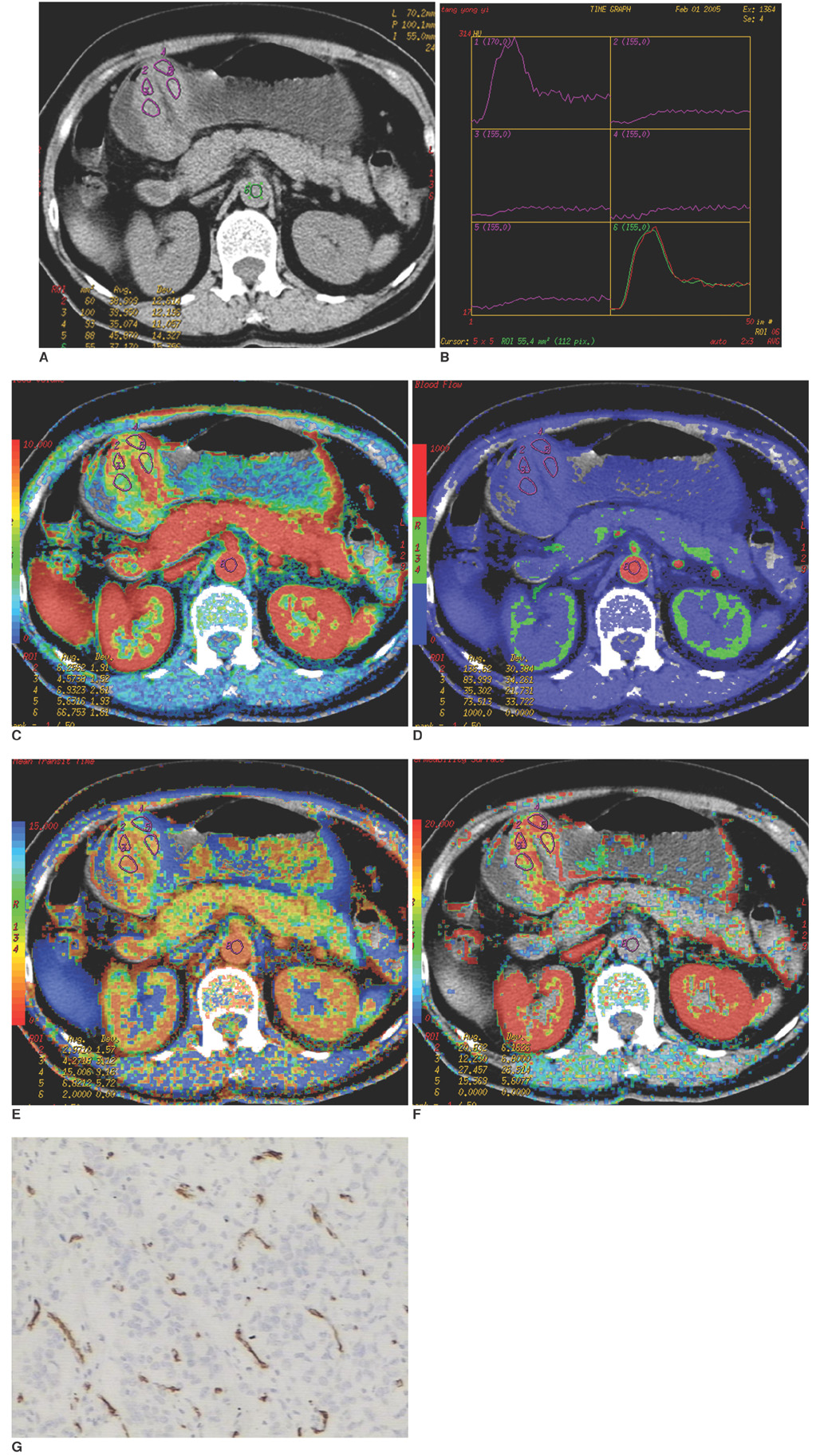
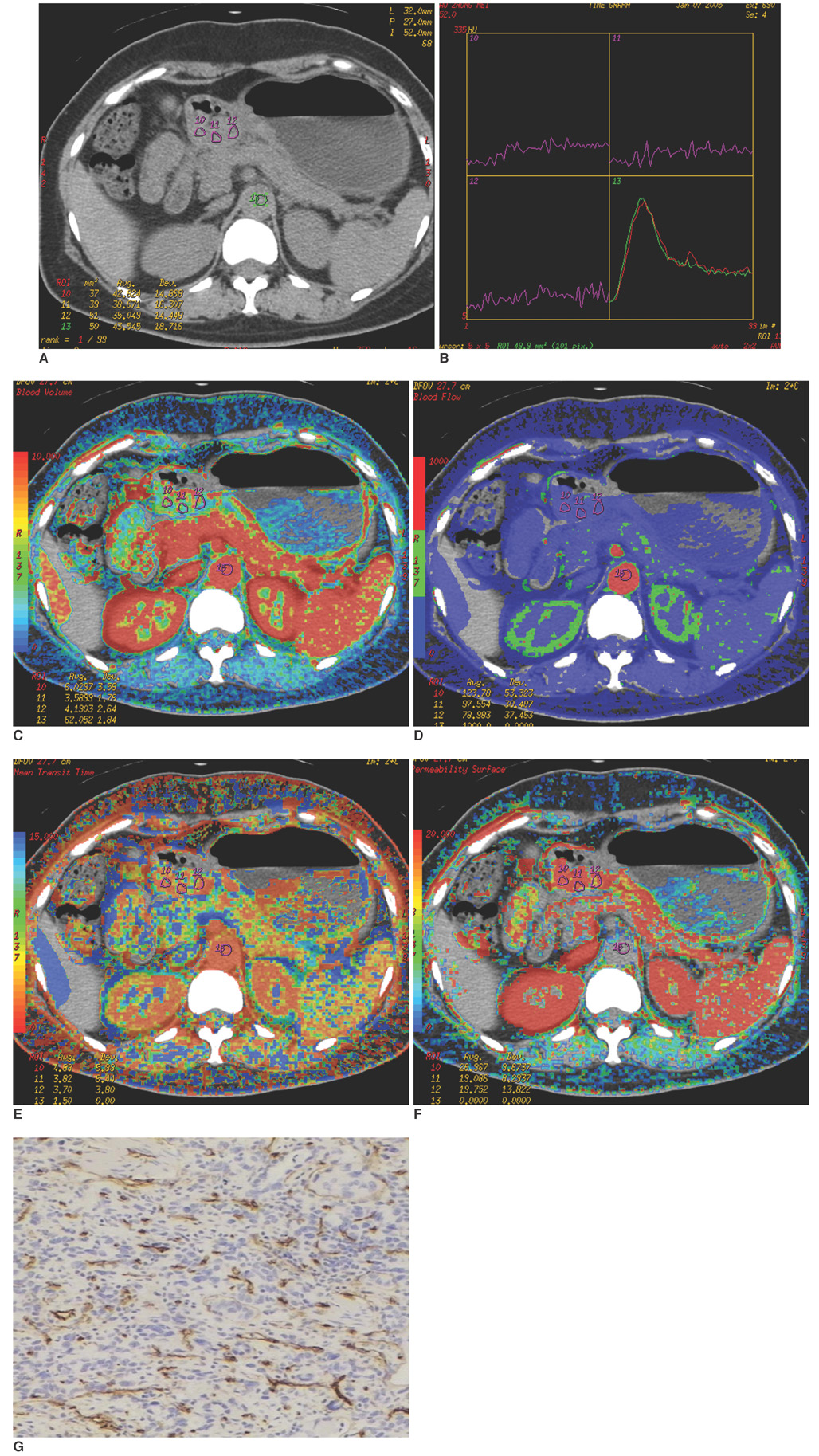
To investigate the relationship between the perfusion CT features and the clinicopathologically determined prognostic factors in advanced gastric cancer cases. MATERIALS AND METHODS: A perfusion CT was performed on 31 patients with gastric cancer one week before surgery using a 16-channel multi-detector CT (MDCT) instrument. The data were analyzed with commercially available software to calculate tumor blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability surface (PS). The microvessel density (MVD), was evaluated by immunohistochemical staining of the surgical specimens with anti-CD34. All of the findings were analyzed prospectively and correlated with the clinicopathological findings, which included histological grading, presence of lymph node metastasis, serosal involvement, distant metastasis, tumor, node, metastasis (TNM) staging, and MVD. The statistical analyses used included the Student's t-test and the Spearman rank correlation were performed in SPSS 11.5. RESULTS: The mean perfusion values and MVD for tumors were as follows: BF (48.14+/-16.46 ml/100 g/min), BV (6.70+/-2.95 ml/100 g), MTT (11.75+/-4.02 s), PS (14.17+/-5.23 ml/100 g/min) and MVD (41.7+/-11.53). Moreover, a significant difference in the PS values was found between patients with or without lymphatic involvement (p = 0.038), as well as with different histological grades (p = 0.04) and TNM stagings (p = 0.026). However, BF, BV, MTT, and MVD of gastric cancer revealed no significant relationship with the clinicopathological findings described above (p > 0.05). CONCLUSION: The perfusion CT values of the permeable surface could serve as a useful prognostic indicator in patients with advanced gastric cancer.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Prediction of Treatment Outcome of Chemotherapy Using Perfusion Computed Tomography in Patients with Unresectable Advanced Gastric Cancer
Dong Ho Lee, Se Hyung Kim, Sang Min Lee, Joon Koo Han
Korean J Radiol. 2019;20(4):589-598. doi: 10.3348/kjr.2018.0306.Dynamic Contrast-Enhanced Ultrasound of Gastric Cancer: Correlation with Perfusion CT and Histopathology
Ijin Joo, Se Hyung Kim, Dong Ho Lee, Joon Koo Han
Korean J Radiol. 2019;20(5):781-790. doi: 10.3348/kjr.2018.0273.
Reference
-
1. Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, et al. Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Zhonghua Zhong Liu Za Zhi. 2004. 26:4–8.2. Ahn MJ, Jang SJ, Park YW, Choi JH, Oh HS, Lee CB, et al. Clinical prognostic values of vascular endothelial growth factor, MVD and p53 expression in esophageal carcinomas. J Korean Med Sci. 2002. 17:201–207.3. Tenderenda M, Rutkowski P, Jesionek-kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001. 7:129–134.4. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971. 285:1182–1186.5. Tuncbilek N, Karakas HM, Altaner S. Dynamic MRI in indirect estimation of MVD, histologic grade, and prognosis in colorectal adenocarcinomas. Abdom Imaging. 2004. 29:166–172.6. Roberts HC, Roberts TP, Lee TY, Dillon W. Dynamic, contrast-enhanced CT of human brain tumors: quantitative assessment of blood volume, blood flow, and microvascular permeability. AJNR Am J Neuroradiol. 2002. 23:828–832.7. Blomley MJ, Coulden R, Bufkin C, Lipton MJ, Dawson P. Contrast bolus dynamic computed tomography for the measurement of solid organ perfusion. Invest Radiol. 1993. 28:suppl 5. S72–S77.8. Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997. 205:471–478.9. Dugdale PE, Miles KA. Hepatic metastases: the value of quantitative assessment of contrast enhancement on computed tomography. Eur J Radiol. 1999. 30:206–213.10. Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005. 234:785–792.11. Hamilton SR. Pathology and genetics of tumors of the digestive system. 2000. Lyon: IARC press;46–48.12. Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, et al. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004. 213:73–82.13. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J. 1991. 324:1–8.14. Miles KA. Perfusion CT for the assessment of tumor vascularity: which protocol? Br J Radiol. 2003. 76:S36–S42.15. Hoeffner EG, Case I, Jain R, Gujar SK, Shah GV, Deveikis JP, et al. Cerebral perfusion CT: technique and clinical applications. Radiology. 2004. 231:632–644.16. Eastwood JD, Lev MH, Provenzale JM. Perfusion CT with iodinated contrast material. AJR Am J Roentgenol. 2003. 180:3–12.17. Harvey CJ, Blomley MJ, Dawson P, Morgan JA, Dooher A, Deponte J, et al. Functional CT imaging of the acute hyperemic response to radiation therapy of the prostate gland: early experience. J Comput Assist Tomogr. 2001. 25:43–49.18. Goh V, Halligan S, Hugill JA, Gartner L, Bartram CI. Quantitative colorectal cancer perfusion measurement using dynamic contrast-enhanced multidetector -row CT. J Comput Assist Tomogr. 2005. 29:59–63.19. Setala LP, Kosma VM, Marin S, Lipponen PK, Eskelinen MJ, Syrjanen KJ, et al. Prognostic factors in gastric cnacer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer. 1996. 74:766–772.20. Gabbert HE, Meier S, Gerharz CD, Hommel G. Tumor cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int J Cancer. 1992. 50:202–207.21. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000. 407:249–257.22. Raghunand N, Gatenby RA, Gillies RJ. Microenviromental and cellular consequences of altered blood flow in tumors. Br J Radiol. 2003. 76:S11–S22.23. Kuszyk BS, Corl FM, Franano FN, Bluemke DA, Hofmann LV, Fortman BJ, et al. Tumor transport physiology: implications for imaging and imaging-guided therapy. AJR Am J Roentgenol. 2001. 177:747–753.24. Li ZP, Meng QF, Sun CH, Xu DS, Fan M, Yang XF, et al. Tumor angiogenesis and dynamic CT in colorectal carcinoma: radiologic-pathologic correlation. World J Gastroenterol. 2005. 11:1287–1291.25. Brix G, Bahner ML, Hoffmann U, Horvath A, Schreiber W. Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT-initial experience. Radiology. 1999. 210:269–276.26. Schmainda KM, Rand SD, Joseph AM, Lund R, Ward BD, Pathak AP, et al. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004. 25:1524–1532.27. Shin JH, Lee HK, Kwun BD, Kim JS, Kang W, Choi CG, et al. Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. AJR Am J Roentgenol. 2002. 179:783–789.28. Abdalla SA, Behzad F, Bsharah S, Kumar S, Amini SK, O'Dwyer ST, et al. Prognostic relevance of MVD in colorectal tumors. Oncol Rep. 1999. 6:839–842.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation Between Functional Myocardial Perfusion Imaging and Anatomical Cardiac CT in a Case of Myocardial Bridging
- Reverse Redistribution in Myocardial Perfusion Imaging: Revisited with 64-slice MDCT
- Multidetector-Row CT Findings of Gastric Cystic Lymphangioma: A Case Report
- Multidetector-row CT of the Gastrointestinal Tract
- MDCT Application of Thoracic Imaging