Clin Orthop Surg.
2011 Dec;3(4):315-324. 10.4055/cios.2011.3.4.315.
Effect of Hyaluronic Acid-Carboxymethylcellulose Solution on Perineural Scar Formation after Sciatic Nerve Repair in Rats
- Affiliations
-
- 1Department of Orthopaedic Surgery, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Department of Orthopaedic Surgery, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea. ljhos69@naver.com
- 3Department of Orthopaedic Surgery, Kyung Hee University School of Medicine, Seoul, Korea.
- 4Department of Pathology, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 1097170
- DOI: http://doi.org/10.4055/cios.2011.3.4.315
Abstract
- BACKGROUND
Scar tissue formation is the major cause of failure in peripheral nerve surgery. Use of a hyaluronic acid-carboxymethylcellulose (HA-CMC) membrane (Seprafilm) as a solid anti-adhesion barrier agent is one of the therapeutic approaches to reduce postoperative scar tissue formation. However, a solid membrane may not be suitable for repair of a weak peripheral nerve site. This study examined the effect of HA-CMC solution on perineural scar formation after peripheral nerve repair in rats.
METHODS
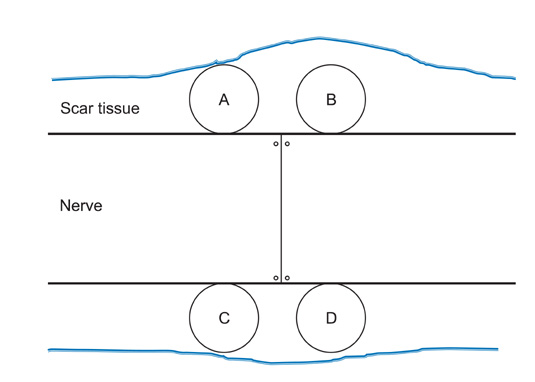
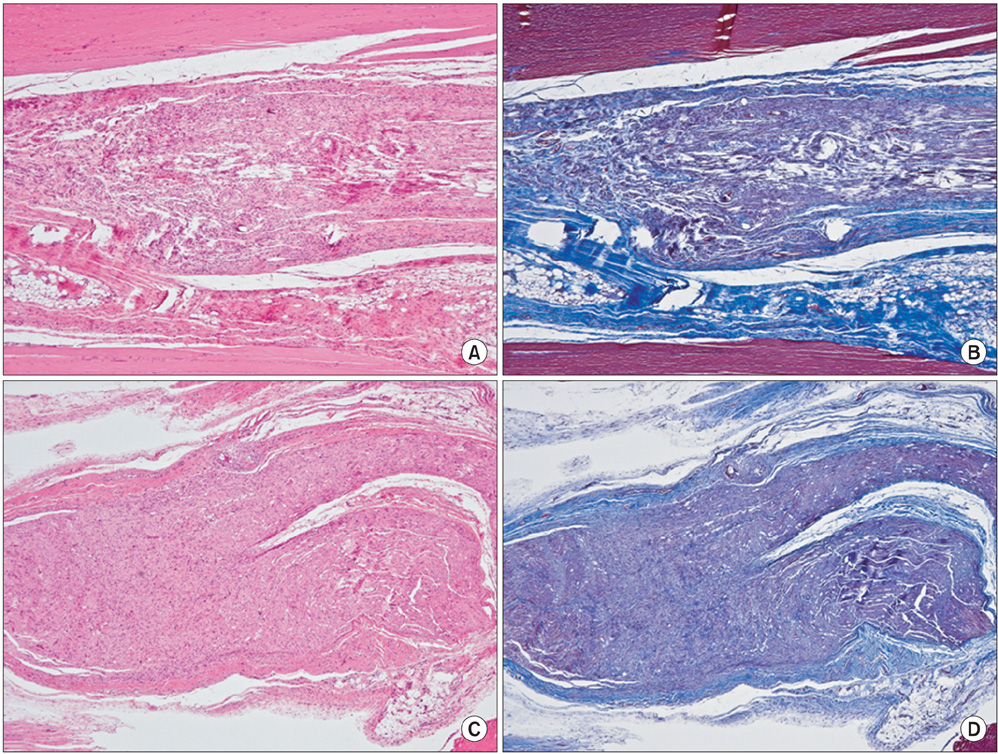
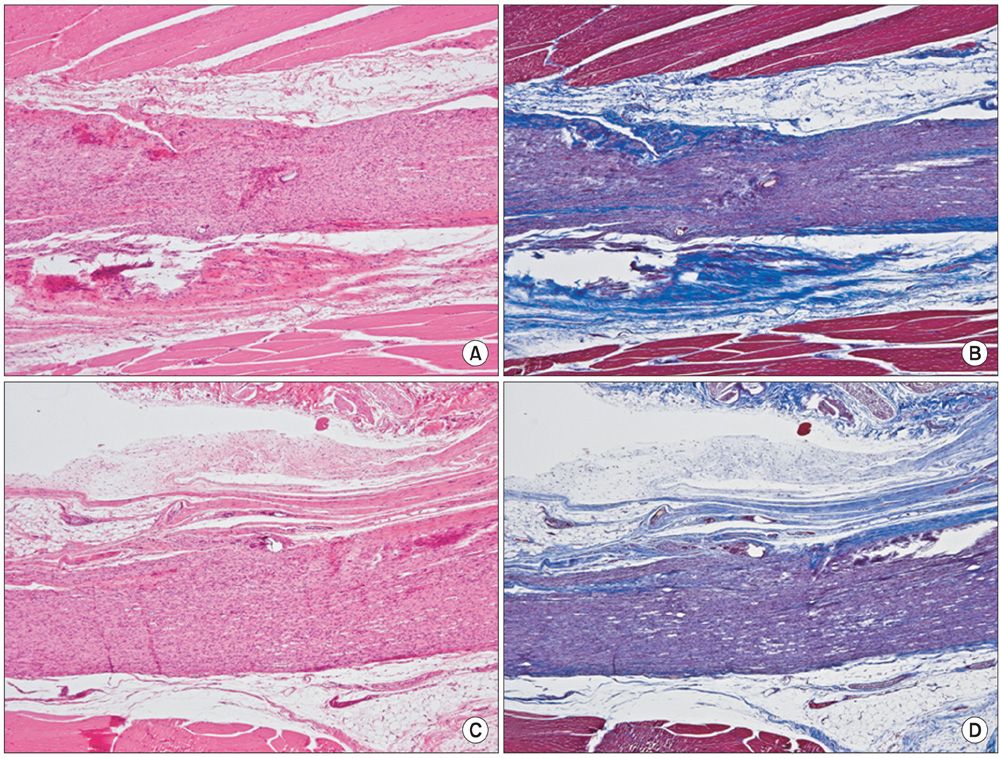
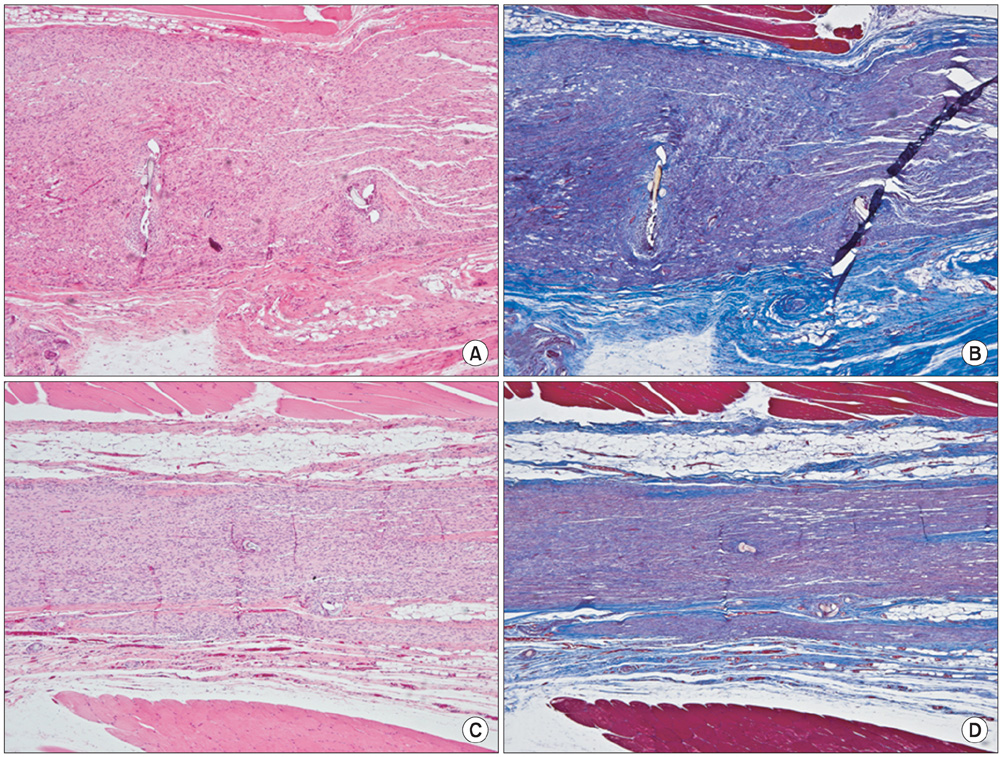
The sciatic nerves of 40 rats were transected and then immediately repaired using 10-0 nylon. The nerves were divided randomly into two groups. Saline and HA-CMC solution were applied topically to the nerve repair sites in the control and experimental groups, respectively. Reoperation was performed at 3, 6, 9, and 12 weeks to assess scar tissue formation. The assessment included the quality of wound healing, presence of perinueral adhesion, cellular components of the scar tissue, thickness of the scar tissue and histomorphological organization of the repair site.
RESULTS
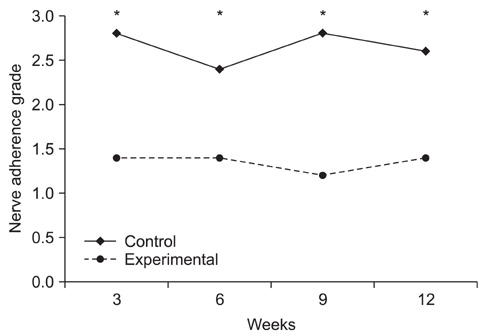
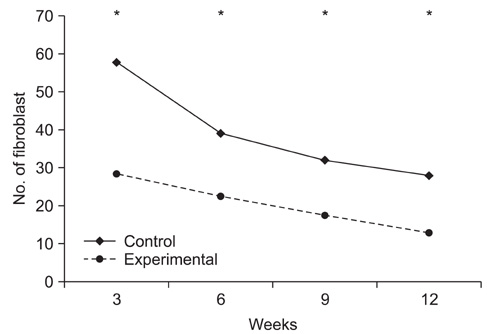
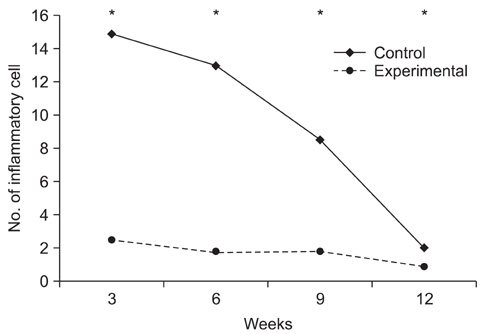
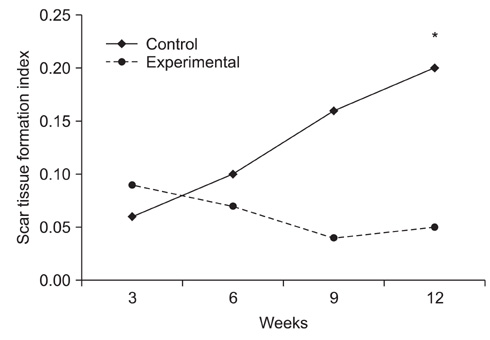
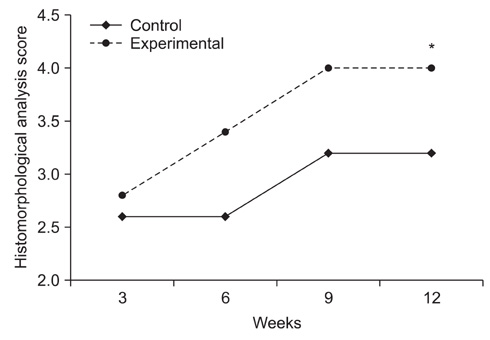
Topical application of the HA-CMC solution significantly decreased the macroscopic nerve adherence score and the numbers of the cellular components such as fibroblasts and inflammatory cells (p < 0.05, Mann-Whitney U-test). The scar tissue formation index was significantly lower in the experimental group at 12 weeks than that in the control group (p < 0.05, Mann-Whitney U-test). The grading scores of the histomorphological axonal organization at the repair site were significantly higher in the experimental group than those in the control group at 12 weeks (p < 0.05, Mann-Whitney U-test). No evidence of wound dehiscence or inflammatory reactions against the HA-CMC solution was noted.
CONCLUSIONS
Topical application of a HA-CMC solution is effective in reducing the perineural scar formation and adhesion after sciatic nerve repair in rats, and is effective in promoting peripheral nerve regeneration at the repair site.
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacologic treatment of osteoarthritis
Seung-Hoon Baek, Shin-Yoon Kim
J Korean Med Assoc. 2013;56(12):1123-1131. doi: 10.5124/jkma.2013.56.12.1123.
Reference
-
1. Rydevik B, Lundborg G, Nordborg C. Intraneural tissue reactions induced by internal neurolysis: an experimental study on the blood-nerve barrier, connective tissues and nerve fibres of rabbit tibial nerve. Scand J Plast Reconstr Surg. 1976. 10(1):3–8.2. Wilgis EF, Murphy R. The significance of longitudinal excursion in peripheral nerves. Hand Clin. 1986. 2(4):761–766.
Article3. Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986. 119(2):219–234.
Article4. Wang KK, Nemeth IR, Seckel BR, et al. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery. 1998. 18(4):270–275.
Article5. Burns JW, Skinner K, Colt J, et al. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995. 59(6):644–652.
Article6. Schimizzi AL, Massie JB, Murphy M, et al. High-molecularweight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J. 2006. 6(5):550–556.
Article7. Ikeda K, Yamauchi D, Osamura N, Hagiwara N, Tomita K. Hyaluronic acid prevents peripheral nerve adhesion. Br J Plast Surg. 2003. 56(4):342–347.
Article8. Ozgenel GY. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003. 23(6):575–581.
Article9. Isik S, Ozturk S, Gurses S, et al. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. Br J Plast Surg. 1999. 52(5):373–379.
Article10. Yamamoto M, Endo N, Ito M, et al. Novel polysaccharide-derived hydrogel prevents perineural adhesions in a rat model of sciatic nerve adhesion. J Orthop Res. 2010. 28(3):284–288.
Article11. Rodgers KE, Robertson JT, Espinoza T, et al. Reduction of epidural fibrosis in lumbar surgery with Oxiplex adhesion barriers of carboxymethylcellulose and polyethylene oxide. Spine J. 2003. 3(4):277–283.
Article12. Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronatebased bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996. 183(4):297–306.13. Adanali G, Verdi M, Tuncel A, Erdogan B, Kargi E. Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J Reconstr Microsurg. 2003. 19(1):29–36.
Article14. Magill CK, Tuffaha SH, Yee A, et al. The short- and long-term effects of Seprafilm on peripheral nerves: a histological and functional study. J Reconstr Microsurg. 2009. 25(6):345–354.
Article15. Lew DH, Yoon JH, Hong JW, Tark KC. Efficacy of antiadhesion barrier solution on periimplant capsule formation in a white rat model. Ann Plast Surg. 2010. 65(2):254–258.
Article16. Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery. 1996. 38(5):976–983.
Article17. Brown RE, Erdmann D, Lyons SF, Suchy H. The use of cultured Schwann cells in nerve repair in a rabbit hind-limb model. J Reconstr Microsurg. 1996. 12(3):149–152.
Article18. Jones NF, Shaw WW, Katz RG, Angeles L. Circumferential wrapping of a flap around a scarred peripheral nerve for salvage of end-stage traction neuritis. J Hand Surg Am. 1997. 22(3):527–535.
Article19. Dumanian GA, McClinton MA, Brushart TM. The effects of free fat grafts on the stiffness of the rat sciatic nerve and perineural scar. J Hand Surg Am. 1999. 24(1):30–36.
Article20. Xu J, Varitimidis SE, Fisher KJ, Tomaino MM, Sotereanos DG. The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. J Hand Surg Am. 2000. 25(1):93–103.
Article21. Finsterbush A, Porat S, Rousso M, Ashur H. Prevention of peripheral nerve entrapment following extensive soft tissue injury, using silicone cuffing: an experimental study. Clin Orthop Relat Res. 1982. (162):276–281.22. Nachemson AK, Lundborg G, Myrhage R, Rank F. Nerve regeneration and pharmacological suppression of the scar reaction at the suture site: An experimental study on the effect of estrogen-progesterone, methylprednisolone-acetate and cis-hydroxyproline in rat sciatic nerve. Scand J Plast Reconstr Surg. 1985. 19(3):255–260.
Article23. Atkins S, Smith KG, Loescher AR, Boissonade FM, Ferguson MW, Robinson PP. The effect of antibodies to TGF-beta1 and TGF-beta2 at a site of sciatic nerve repair. J Peripher Nerv Syst. 2006. 11(4):286–293.
Article24. Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol. 2007. 68(6):615–622.
Article25. Albayrak BS, Ismailoglu O, Ilbay K, et al. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: outcome based on gross postsurgical, histopathological, and ultrastructural findings. J Neurosurg Spine. 2010. 12(3):327–333.
Article26. Hiro D, Ito A, Matsuta K, Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986. 140(2):715–722.
Article27. Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987. 30(7):769–778.
Article28. Dam-Hieu P, Lacroix C, Said G, Devanz P, Liu S, Tadie M. Reduction of postoperative perineural adhesions by Hyaloglide gel: an experimental study in the rat sciatic nerve. Neurosurgery. 2005. 56:2 Suppl. 425–433.
Article29. Gago LA, Saed GM, Chauhan S, Elhammady EF, Diamond MP. Seprafilm (modified hyaluronic acid and carboxymethylcellulose) acts as a physical barrier. Fertil Steril. 2003. 80(3):612–616.
Article30. Klingler PJ, Floch NR, Seelig MH, Branton SA, Wolfe JT, Metzger PP. Seprafilm-induced peritoneal inflammation: a previously unknown complication: report of a case. Dis Colon Rectum. 1999. 42(12):1639–1643.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Study of Scar Formation at the Site of Sciatic Nerve Repair in Rats
- Antiadhesive Effect of the Mixed Solution of Hyaluronate and Sodium Carboxymethylcellulose After Strabismus Surgery
- Arthroscopic Treatment of Secondary Piriformis Syndrome by Perineural Cyst on the Sciatic Nerve: A Case Report
- Comparative study of repair methods in peripheral nerve injury: An experimental study in sciatic nerve of rats
- Histological and electrophysiological study on nerve regenerationfollowing nerve repair in rat sciatic nerve