Korean J Lab Med.
2009 Aug;29(4):353-360. 10.3343/kjlm.2009.29.4.353.
Analysis of Characteristics of Mononuclear Cells Remaining in the Leukoreduction System Chamber of Trima Accel(R) and Their Differentiation Into Dendritic Cells
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. hyunok1019@yuhs.ac
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Yonsei Cell Therapy Center, Seoul, Korea.
- 4Blood Center, Korean Red Cross, Seoul, Korea.
- KMID: 1096930
- DOI: http://doi.org/10.3343/kjlm.2009.29.4.353
Abstract
-
BACKGROUND: We investigated the characteristics of the mononuclear cells remaining in the leukoreduction system (LRS) chambers of Trima Accel(R) in comparison with those of standard buffy coat cells, and evaluated their potential for differentiation into dendritic cells.
METHODS
Twenty-six LRS chambers of Trima Accel(R) were collected after platelet pheresis from healthy adults. Flow cytometric analysis for T, B, NK, and CD14+ cells was performed and the number of CD34+ cells was counted. Differentiation and maturation into dendritic cells were induced using CD14+ cells seperated via Magnetic cell sorting (MACS(R)) Seperation (Miltenyi Biotec Inc., USA).
RESULTS
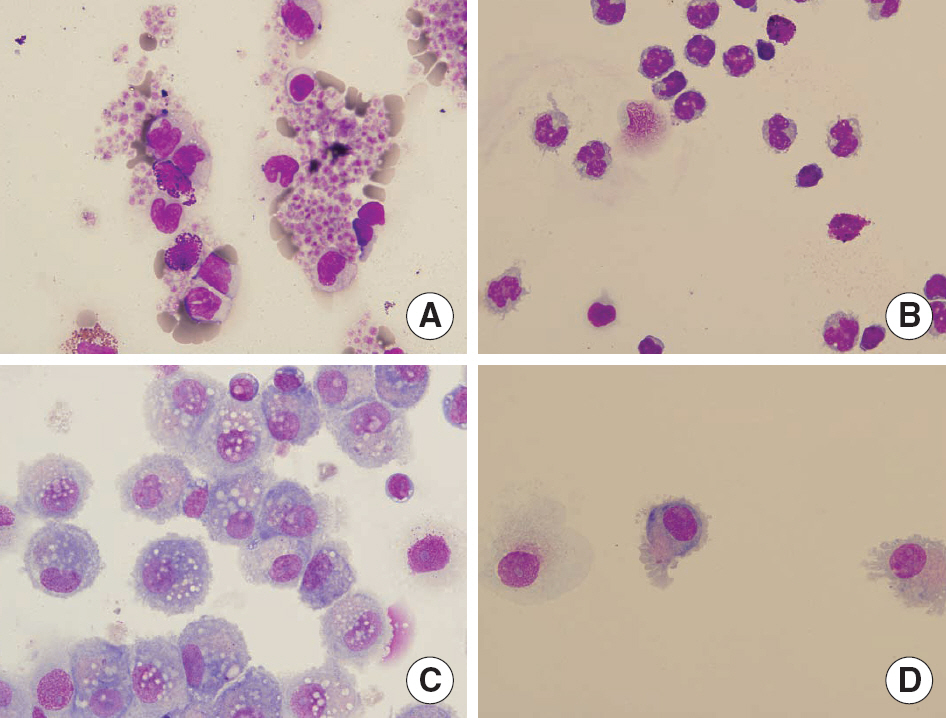
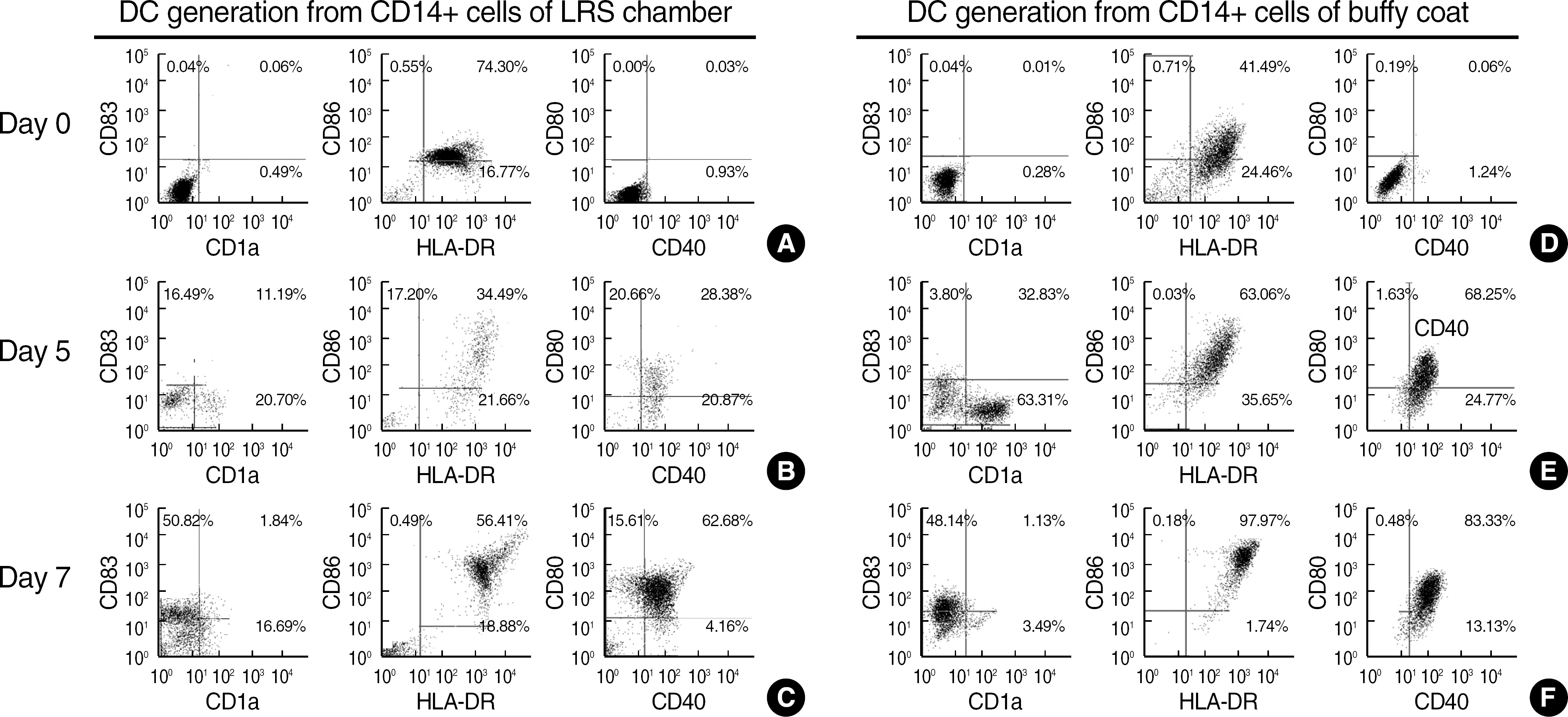
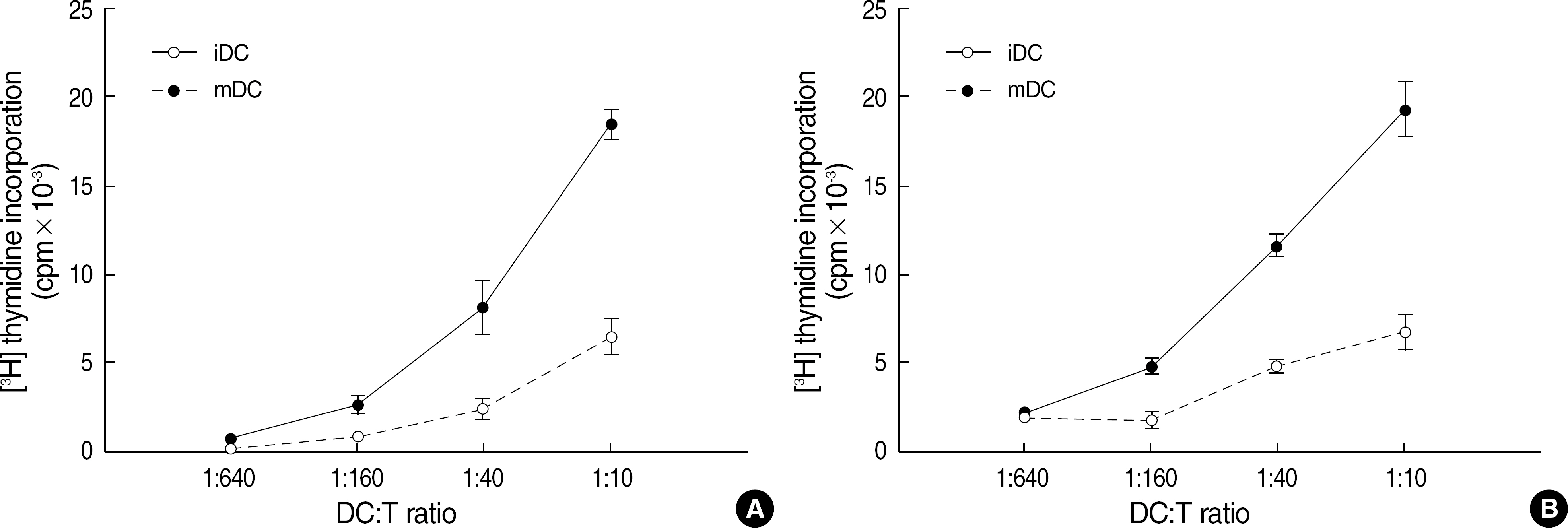
Total white blood cell (WBC) count in LRS chambers was 10.8x108 (range 7.7-18.0x108). The median values (range) of proportions of each cells were CD4+ T cell 29.6% (18.7-37.6), CD8+ T cell 27.7% (19.2-40.0), B cell 5.5% (2.2-12.1), NK cell 15.7% (13.7-19.9), and CD14+ cells 12.4% (8.6-32.3) respectively. Although total WBC count was significantly higher in the buffy coat (whole blood of 400 mL) than the LRS chambers, the numbers of lymphocytes and monocytes were not statistically different. The numbers of B cells and CD4+ cells were significantly higher in the buffy coat than the LRS chambers (P<0.05). The median value (range) of CD34+ cells obtained from the LRS chambers was 0.9x10(6) (0.2-2.6x10(6)). After 7 days of cytokine-supplemented culture, the CD14+ cells were successfully differentiated into dendritic cells.
CONCLUSIONS
The mononuclear cells in LRS chambers of Trima Accel(R) are an excellent alternative source of viable and functional human blood cells, which can be used for research purposes.
Keyword
MeSH Terms
Figure
Reference
-
1.Shehata N., Lin Y., Pendergrast J., Branch DR. Cellular therapies: a Canadian blood services research and development symposium. Transfus Med Rev. 2007. 21:317–36.
Article2.Mellman I., Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001. 106:255–8.3.Sniecinski I., O'Donnell MR., Nowicki B., Hill LR. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988. 71:1402–7.
Article4.Meyer TP., Zehnter I., Hofmann B., Zaisserer J., Burkhart J., Rapp S, et al. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005. 307:150–66.
Article5.Ebner S., Neyer S., Hofer S., Nussbaumer W., Romani N., Heufler C. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001. 252:93–104.
Article6.Adams MR., Dumont LJ., McCall M., Heaton WA. Clinical trial and local process evaluation of an apheresis system for preparation of white cell-reduced platelet components. Transfusion. 1998. 38:966–74.
Article7.Neron S., Thibault L., Dussault N., Cote G., Ducas E., Pineault N, et al. Characterization of mononuclear cells remaining in the leukoreduction system chambers of apheresis instruments after routine platelet collection: a new source of viable human blood cells. Transfusion. 2007. 47:1042–9.
Article8.Dietz AB., Bulur PA., Emery RL., Winters JL., Epps DE., Zubair AC, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006. 46:2083–9.
Article9.Karimi K., Boudreau JE., Fraser K., Liu H., Delanghe J., Gauldie J, et al. Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFN gamma-producing NK cells. Mol Ther. 2007. 16:411–8.10.Lee JJ., Nam CE., Nam JH., Lee HC., Chung IJ., Park MS, et al. Generation of cytotoxic donor CD8+ T cells against relapsing leukemic cells following allogeneic transplantation by stimulation with leukemic cell- or leukemic lysate pulsed donor cell-derived dendritic cells. Leuk Res. 2004. 28:517–24.
Article11.Weitkamp JH., Crowe JE Jr. Blood donor leukocyte reduction filters as a source of human B lymphocytes. Biotechniques. 2001. 31:464–6.
Article12.Teleron AA., Carlson B., Young PP. Blood donor white blood cell reduction filters as a source of human peripheral blood-derived endothelial progenitor cells. Transfusion. 2005. 45:21–5.
Article13.Ivanovic Z., Duchez P., Morgan DA., Hermitte F., Lafarge X., Chevaleyre J, et al. Whole-blood leuko-depletion filters as a source of CD 34+ progenitors potentially usable in cell therapy. Transfusion. 2006. 46:118–25.14.Pickl WF., Majdic O., Kohl P., Stockl J., Riedl E., Scheinecker C, et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996. 157:3850–9.15.Reddy A., Sapp M., Feldman M., Subklewe M., Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997. 90:3640–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of Isolation Processing of Monocytes from Peripheral Blood Mononuclear Cells and Differentiation into Dendritic Cells
- Malignant histiocytosis: Clinicopathologic review of 18 cases with immunohistochemical study
- Regulation of Osteoclast Differentiation: Identification of osteoclast and macrophage fusion protein; DC-STAMP
- Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice
- Characterization of the Proliferated Histiocytes in Acute Leukemia by Performing Immunohistochemistry