J Vet Sci.
2007 Sep;8(3):255-261. 10.4142/jvs.2007.8.3.255.
Variation in the molecular weight of Photobacterium damselae subsp. piscicida antigens when cultured under different conditions in vitro
- Affiliations
-
- 1Laboratory of Fish and Shellfish Diseases, College of Veterinary Medicine, Gyeongsang National University, Jinju 660-701, Korea. jungts@gsnu.ac.kr
- 2Aquatic Vaccine Unit, Institute of Aquaculture, University of Stirling, Stirling, FK9 4LA, Scotland, UK.
- 3Dipartimento di Scienze della Produzione Animale, Faculta Di Medicina Veterinaria, Universita degli studi di Udine, 20B 33100 Udine, Italy.
- KMID: 1090800
- DOI: http://doi.org/10.4142/jvs.2007.8.3.255
Abstract
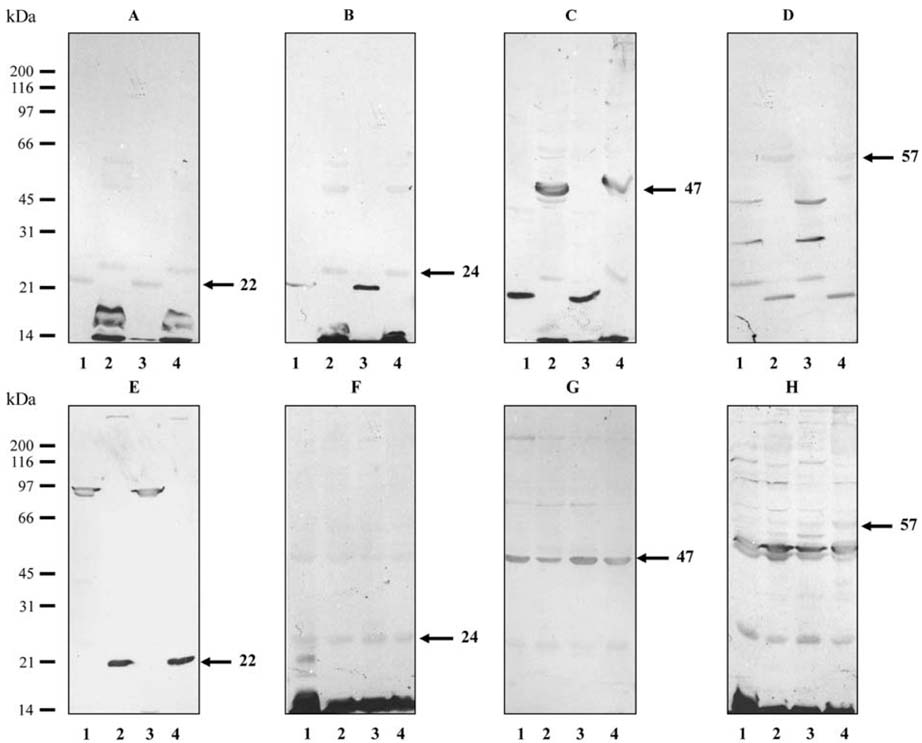
- The antigenicity of Photobacterium damselae (Ph. d.)subsp. piscicida, cultured in four different growth media[tryptone soya broth (TSB), glucose-rich medium (GRM),iron-depleted TSB (TSB+IR-), and iron-depleted GRM(GRM+IR-)] was compared by enzyme-linked immuno-sorbent assay (ELISA) and Western blot analysis usingsera obtained from sea bass (Dicentrarchus labrax) raisedagainst live or heat-killed Ph. d. subsp. piscicida. Theantigenic expression of Ph. d. subsp. piscicida was found todiffer depending on the culture medium used. A significantlyhigher antibody response was obtained with iron-depletedbacteria by ELISA compared with non-iron depletedbacteria obtained from the sera of sea bass raised againstlive Ph. d. subsp. piscicida. The sera from sea bass raisedagainst live bacteria showed a band at 22kDa in bacteriacultured in TSB+IR- or GRM+IR- when bacteria thathad been freshly isolated from fish were used for thescreening, while bands at 24 and 47kDa were observedwith bacteria cultured in TSB or GRM. When bacteriawere passaged several times on tryptic soya agar prior toculturing in the four different media, only bands at 24 and47kDa were recognized, regardless of the medium used toculture the bacteria. It would appear that the molecularweight of Ph. d. subsp. piscicida antigens change in thepresence of iron restriction, and sera from sea bassinfected with live bacteria are able to detect epitopes onthe antigens after this shift in molecular weight.
Keyword
MeSH Terms
-
Animals
Antibodies, Bacterial/blood
Antigens, Bacterial/immunology/*metabolism
Bass/blood/*immunology
Blotting, Western/veterinary
Cell Count/methods
Culture Media
Enzyme-Linked Immunosorbent Assay/veterinary
Fish Diseases/immunology/*microbiology
Molecular Weight
Pasteurella Infections/immunology/microbiology/*veterinary
Photobacterium/*immunology
Figure
Cited by 1 articles
-
In vivo morphological and antigenic characteristics of Photobacterium damselae subsp. piscicida
Tae S. Jung, Kim D. Thompson, Donatella Volpatti, Marco Galeotti, A. Adams
J Vet Sci. 2008;9(2):169-175. doi: 10.4142/jvs.2008.9.2.169.
Reference
-
1. Arijo S, Balebona C, Martinez-Manzanares E, Moriñigo MA. Immune response of gilt-head seabream (Sparus aurata) to antigens from Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2004. 16:65–70.
Article2. Bakopoulos V, Adams A, Richards RH. The effect of iron limitation growth conditions on the cell and extracellular components of the fish pathogen Pasteurella piscicida. J Fish Dis. 1997. 20:297–305.
Article3. Bakopoulos V, Hanif A, Poulos K, Galeotti M, Adams A, Dimitriadis GJ. The effect of in vivo growth on the cellular and extracellular components of the marine bacterial pathogen Photobacterium damsela subsp. piscicida. J Fish Dis. 2004. 27:1–13.
Article4. Bakopoulos V, Pearson M, Volpatti D, Gousmani L, Adams A, Galeotti M, Dimitriadis GJ. Investigation of media formulations promoting differential antigen expression by Photobacterium damsela ssp. piscicida and recognition by sea bass, Dicentrarchus labrax (L.), immune sera. J Fish Dis. 2003. 26:1–13.
Article5. Bakopoulos V, Volpatti D, Adams A, Galeotti M, Richards R. Qualitative differences in the immune response of rabbit, mouse and sea bass, Dicentrarchus labrax, L. to Photobacterium damsela subsp. piscicida, the causative agent of fish Pasteurellosis. Fish Shellfish Immunol. 1997. 7:161–174.
Article6. Bakopoulos V, Volpatti D, Gusmani L, Galeotti M, Adams A, Dimitriadis GJ. Vaccination trials of sea bass, Dicentrarchus labrax (L.), against Photobacterium damsela subsp. piscicida, using novel vaccine mixtures. J Fish Dis. 2003. 26:77–90.
Article7. Balebona MC, Zorrilla I, Morinigo MA, Borrego JJ. Survey of bacterial pathologies affecting farmed gilt-head sea bream (Sparus aurata L.) in southwestern Spain from 1990 to 1996. Aquaculture. 1998. 166:19–35.
Article8. Bollag DM, Rozycki MD, Edelstein SJ. Protein Methods. 1996. 2nd ed. New York: Wiley-Liss.9. Bonet R, Magarinos B, Romalde JL, Simon-Pujol MD, Toranzo AE, Congregado F. Capsular polysaccharide expressed by Pasteurella piscicida grown in vitro. FEMS Microbiol Lett. 1994. 124:285–289.
Article10. Díaz-Rosales P, Chabrillón M, Moriñigo MA, Balebona MC. Survival against exogenous hydrogen peroxide of Photobacterium damselae subsp. piscicida under different culture conditions. J Fish Dis. 2003. 26:305–308.11. do Vale A, Magarinos B, Romalde JL, Lemos ML, Ellis AE, Toranzo AE. Binding of haemin by the fish pathogen Photobacterium damselae subsp. piscicida. Dis Aquat Organ. 2002. 48:109–115.
Article12. Ellis AE, Stapleton KJ, Hastings TS. The humoral immune response of rainbow trout (Salmo gairdneri) immunised by various regimes and preparations of Aeromonas salmonicida antigens. Vet Immunol Immunopathol. 1988. 19:153–164.
Article13. Hawke JP, Thune RL, Cooper RK, Judice E, Kelly-Smith M. Molecular and phenotypic characterization of strains of Photobacterium damselae subsp. piscicida isolated from hybrid striped bass cultured in Louisiana, USA. J Aquat Anim Health. 2003. 15:189–201.
Article14. Hirst ID, Ellis AE. Iron-regulated outer membrane proteins of Aeromonas salmonicida are important protective antigens in Atlantic salmon against furunculosis. Fish Shellfish Immunol. 1994. 4:29–45.
Article15. Kawakami H, Sakai M. Comparison of susceptibility of seven fishes to Photobacterium damsela subsp. piscicida. Bull Eur Assoc Fish Pathol. 1999. 19:153–155.16. Kawakami H, Shinohara N, Fukuda Y, Yamashita H, Kihara H, Sakai M. The efficacy of lipopolysaccharide mixed chloroform-killed cell (LPS-CKC) bacterin of Pasteurella piscicida on Yellowtail, Seriola quinqueradiata. Aquaculture. 1997. 154:95–105.
Article17. Kawano K, Aoki , Kitao T. Duration of protection against vibriosis in ayu Plecoglossus altivelis vaccinated by immersion and oral administration with Vibrio anguillarum. Bull Jpn Soc Sci Fish. 1984. 50:771–774.
Article18. Kusuda R, Fukuda Y. Agglutinating antibody titers and serum protein changes of yellowtail after immunization with Pasteurella piscicida cells. Bull Jpn Soc Sci Fish. 1980. 46:969–973.19. Kusuda R, Kawai K. Bacterial diseases of cultured marine fish in Japan. Fish Pathol. 1998. 33:221–227.
Article20. Kusuda R, Ninomiya M, Hamaguchi M, Muraoka A. The efficacy of ribosomal vaccine prepared from Pasteurella piscicida against pseudotuberculosis in cultured yellowtail. Fish Pathol. 1988. 23:191–196.
Article21. Magariños B, Bonet R, Romalde JL, Martínez MJ, Congregado F, Toranzo AE. Influence of the capsular layer on the virulence of Pasteurella piscicida for fish. Microb Pathog. 1996. 21:289–297.
Article22. Magariños B, Romalde JL, Lemos ML, Barja JL, Toranzo AE. Iron uptake by Pasteurella piscicida and its role in pathogenicity for fish. Appl Environ Microbiol. 1994. 60:2990–2998.
Article23. Matsubara A, Mihara S, Kusuda R. Assay of Pasteurella piscicida lipopolysaccharide specific serum antibody in Seriola quinqueradiata by ELISA technique. Bull Jpn Soc Sci Fish. 1985. 51:927–932.24. Mazzolini E, Ceschia G, Giorgetti G, Magni A, Passera A, Danielis L, Marotta A. Caratteristiche fisiche ed immunoreattive dei sieri di branzini (Dicentrarchus labrax) sensibilizzati con Pasteurella piscicida. Boll Soc Ital Patol Ittica. 1996. 19:12–21.25. Moriñigo MA, Romalde JL, Chabrillon M, Magariños B, Arijo S, Balebona MC, Toranzo AE. Effectiveness of a divalent vaccine for gilt-head sea bream (Sparus aurata) against Vibrio alginolyticus and Photobacterium damselae subsp. piscicida. Bull Eur Assoc Fish Pathol. 2002. 22:298–303.26. Nitzan S, Shwartsburd B, Heller ED. The effect of growth medium salinity of Photobacterium damselae subsp. piscicida on the immune response of hybrid bass (Morone saxatilis x M. chrysops). Fish Shellfish Immunol. 2004. 16:107–116.
Article27. Nitzan S, Shwartsburd B, Vaiman R, Heller ED. Some characteristics of Photobacterium damselae ssp. piscicida isolated in Israel during outbreaks of pasteurellosis in hybrid bass (Morone saxatilis x M. chrysops). Bull Eur Assoc Fish Pathol. 2001. 21:77–80.28. Noya M, Magarinos B, Lamas J. Interactions between peritoneal exudate cells (PECs) of gilthead seabream (Sparus aurata) and Pasteurella piscicida. A morphological study. Aquaculture. 1995. 131:11–21.
Article29. Santos Y, Bandin I, Nuñez S, Gravningen K, Toranzo AE. Protection of turbot, Scophthalmus maximus (L.), and rainbow trout, Oncorhynchus mykiss (Richardson), against vibriosis using two different vaccines. J Fish Dis. 1991. 14:407–411.
Article30. Satoh KI, Fukuda Y, Nakano S. Changes in agglutinin titer against Pasteurella piscicida in cultured yellowtail during the epizootics of pseudotuberculosis in 1993 and 1994. Fish Pathol. 1995. 30:291–292.
Article31. Snieszko SF, Bullock GL, Hollis E, Boone JG. Pasteurella sp. from an epizootic of white perch (Roccus americanus) in Chesapeake Bay tidewater areas. J Bacteriol. 1964. 88:1814–1815.
Article32. Thune RL, Fernandez DH, Hawke JP, Miller R. Construction of a safe, stable, efficacious vaccine against Photobacterium damselae ssp. piscicida. Dis Aquat Organ. 2003. 57:51–58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo morphological and antigenic characteristics of Photobacterium damselae subsp. piscicida
- Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria
- A Case with Upper Extremity Deep Vein Thrombosis after in vitro Fertilization
- Comparative antibody response of five recombinant antigens in related to bacterial shedding levels and development of serological diagnosis based on 35 kDa antigen for Mycobacterium avium subsp. paratuberculosis
- Identification of surface antigens of Trichomonas vaginalis