J Vet Sci.
2006 Sep;7(3):293-295. 10.4142/jvs.2006.7.3.293.
Production of monoclonal antibodies against serum immunoglobulins of black rockfish (Sebastes schlegeli Higendorf)
- Affiliations
-
- 1Laboratory of Fish & Shellfish Diseases, College of Veterinary Medicine, Gyeongsang National University, Jinju 660-701, Korea. jungts@gsnu.ac.kr
- 2Gyeongsangnam-do Fisheries Resources Research Institute, Tongyeong 650-947, Korea.
- KMID: 1089916
- DOI: http://doi.org/10.4142/jvs.2006.7.3.293
Abstract
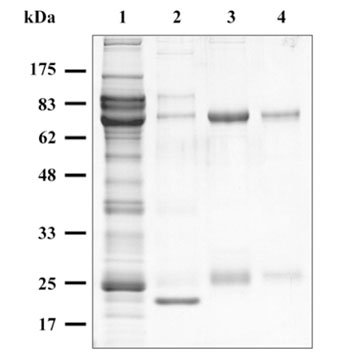
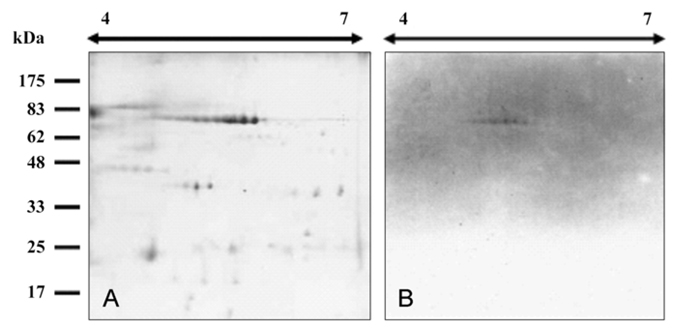
- The present study was undertaken to produce monoclonal antibodies (MAbs) against immunoglobulin (Ig) purified from black rockfish (Sebastes schlegeli Higendorf) serum using protein A, mannan binding protein, and goat IgG affinity columns. These three different ligands were found to possess high affinity for black rockfish serum Ig. All of the Igs purified eluted at only 0.46 M NaCl concentration in anion exchange column chromatography and consisted of two bands at 70 kDa and 25 kDa in SDS-PAGE; they also had similar antigenicity for MAbs to Ig heavy chain in immunoblot assays. Therefore, black rockfish Ig is believed to exist as a single isotype within serum. The MAbs produced against Ig heavy chain reacted specifically with spots distributed over the pI range from 4.8 to 5.6 with a molecular weight of 70 kDa on two dimensional gel electrophoresis immunoblot profiles.
MeSH Terms
-
Animals
Antibodies, Monoclonal/*biosynthesis/immunology
Chromatography, Affinity/veterinary
Electrophoresis, Gel, Two-Dimensional/veterinary
Immunoblotting/veterinary
Immunoglobulin Heavy Chains/immunology
Immunoglobulin Light Chains/immunology
Immunoglobulins/blood/*immunology
Perciformes/blood/*immunology
Figure
Reference
-
1. Al-Harbi AH, Truax R, Thune RL. Production and characterization of monoclonal antibodies against tilapia Oreochromis niloticus immuno globulin. Aquaculture. 2000. 188:219–227.
Article2. Bang JD, Kim JW, Lee SD, Park SI, Chun SG, Jeong CS, Park JW. Humoral immune response of flounder to Edwardsiella tarda: the presence of various sizes of immunoglobulins in flounder. Dis Aquat Organ. 1996. 26:197–203.
Article3. Clem LW. Phylogeny of immuno globulin structure and function. IV. Immunoglobulins of the giant grouper, Epinephelus itaira. J Biol Chem. 1971. 246:9–15.4. Crosbie PBB, Nowak BF. Production of polyclonal antisera against barramundi (Lates calcarifer) serum immunoglobulin derived from affinity columns containing mannan-binding protein or staphylococcal protein A. Aquaculture. 2002. 211:49–63.
Article5. Elcombe BM, Chang RJ, Taves CJ, Winkelhake JL. Evolution of antibody structure and effector functions: comparative hemolytic activities of monomeric and tetrameric IgM from rainbow trout, Salmo gairdnerii. Comp Biochem Physiol B. 1985. 80:697–706.
Article6. Hordvik I, Berven F, Solem S, Hatten F, Endresen C. Analysis of two IgM isotypes in Atlantic salmon and brown trout. Mol Immunol. 2002. 39:313–321.
Article7. Kaattari SL, Piganelli D. Iwama G, Nakanishi T, editors. The specific immune system: Humoral defence. The Fish immune system: Organism, Pathogen, and Environment. 1996. San Diego: Academic Press;207–254.8. Kang SH, Shin GW, Shin YS, J PK, Kim YR, Yang HH, Lee EY, Lee EG, Huh NE, Ju OM, Jung TS. Experimental evaluation of pathogenicity of Lactococcus garvieae in black rockfish (Sebastes schlegeli). J Vet Sci. 2004. 5:387–390.
Article9. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol. 2005. 174:2453–2455.10. Lobb CJ, Olson MO. Immuno-globulin heavy H chain isotypes in a teleost fish. J Immunol. 1988. 141:1236–1245.11. Nevens JR, Mallia AK, Wendt MW, Smith PK. Affinity chromatographic purification of immunoglobulin M antibodies utilizing immobilized mannan binding protein. J Chromatogr. 1992. 597:247–256.
Article12. Palenzuela O, Sitja-Bobadilla A, Alvarez-Pellitero P. Isolation and partial characterization of serum immunoglobulins from sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol. 1996. 6:81–94.
Article13. Park SW, Kim YG, Choi DL. Vibrio ordalii, the causative agent of massive mortality in cultured rockfish (Sebastes schlegeli) larvae. J Fish Pathol. 1996. 9:137–145.14. Pucci B, Coscia MR, Oreste U. Characterization of serum immunoglobulin M of the Antarctic teleost Trematomus bernacchii. Comp Biochem Physiol B Biochem Mol Biol. 2003. 135:349–357.
Article15. Rombout JH, Taverne N, van de Kamp M, Taverne-Thiele AJ. Differences in mucus and serum immunoglobulin of carp (Cyprinus carpio L.). Dev Comp Immunol. 1993. 17:309–317.
Article16. Savan R, Aman A, Sato K, Yamaguchi R, Sakai M. Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol. 2005. 35:3320–3331.
Article17. Seng LT, Colorni A. Woo PTK, Bruno DW, Lim LHS, editors. Infectious diseases of warmwater fish in marine and brackish water. Diseases and Disorders of Finfish in Cage Culture. 2002. Wallingford: CABI Publishing;193–230.18. Tissot JD, Schifferli JA, Hochstrasser DF, Pasquali C, Spertini F, Clement F, Frutiger S, Paquet N, Hughes GJ, Schneider P. Two-dimensional polyacrylamide gel electrophoresis analysis of cryoglobulins and identification of an IgM-associated peptide. J Immunol Methods. 1994. 173:63–75.
Article19. Tizard IR. Veterinary Immunology: an Introduction. 2000. 6th ed. Philadelphia: Saunders;139–148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental evaluation of pathogenicity of Lactococcus garvieae in black rockfish (Sebastes schlegeli)
- First Report of Clavinema mariae (Nematoda: Philometridae) in Cultured Rockfish, Sebastes schlegeli, in Cheonsuman (Bay), the Republic of Korea
- Molecular characterization and genogrouping of VP1 of aquatic birnavirus GC1 isolated from rockfish Sebastes schlegeli in Korea
- Production and characterization of monoclonal antibodies to perchloric acid soluble antigen of M. tuberculosis(TB-II)
- Immunoglobulins for Prophylaxis or Treatment of Infectious Diseases