J Vet Sci.
2006 Dec;7(4):361-368. 10.4142/jvs.2006.7.4.361.
Effects of DDA, CpG-ODN, and plasmid-encoded chicken IFN-gamma on protective immunity by a DNA vaccine against IBDV in chickens
- Affiliations
-
- 1School of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Chuncheon 200-701, Korea. kwonhm@kangwon.ac.kr
- KMID: 1089484
- DOI: http://doi.org/10.4142/jvs.2006.7.4.361
Abstract
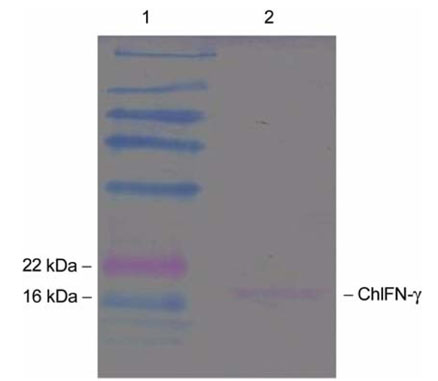
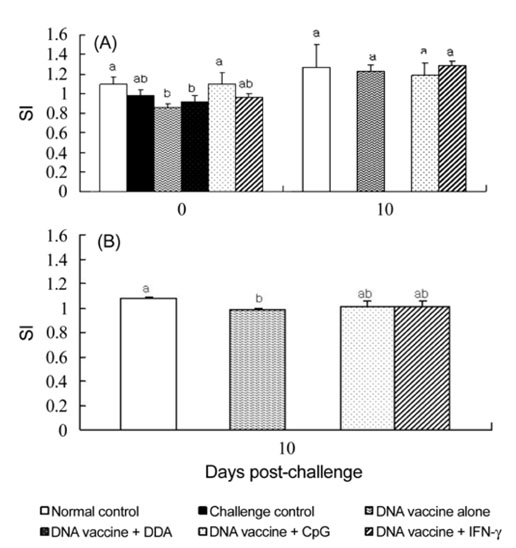
- This study examined the adjuvant effects of dimethyl dioctadecyl ammonium bromide (DDA), CpG oligodeoxynucleotides (CpG-ODN), and chicken interferon-gamma (ChIFN-gamma) on a DNA vaccine (pcDNA-VP243) against the infectious bursal disease virus (IBDV). A plasmid encoding chicken IFN-atilde was constructed. Twice at 2-week intervals, twoweek-old chickens were injected intramuscularly and intraperitoneally with either a DNA vaccine alone or a DNA vaccine together with the respective adjuvants. On week 2 after the second immunization, the chickens were orally challenged with the highly virulent IBDV. The groups that received the DNA vaccines plus either DDA or CpG-ODN showed significantly lower survival rates than the group that received the DNA vaccine alone. However, the survival rates for the DNA vaccine alone and for the DNA vaccine plus ChIFN-gamma were similar. The chickens had no detectable antibodies to the IBDV before the challenge but all the surviving chickens in all groups except for the normal control group showed the induction of antibodies to the IBDV at day 10 after the challenge. As judged by the lymphocyte proliferation assays using the a WST-8 solution performed on the peripheral blood and splenic lymphocytes, the stimulation indices (SI) of the peripheral blood lymphocytes in all groups except for the normal control group were similar immediately before the challenge. At 10 days post-challenge, the SI for DNA vaccine plus either CpG-ODN or ChIFN-gamma was similar to that of the DNA vaccine control group. For splenic lymphocytes, the SI in the DNA vaccine plus CpG-ODN and DNA vaccine plus ChIFN-gamma groups were higher than for the DNA vaccine control. These results suggest that DDA actually compromises the protection against the IBDV by DNA vaccine, and CpG-ODN and IFN-gamma had no significant effect.
MeSH Terms
-
Adjuvants, Immunologic
Animals
Antibodies, Viral/blood
Birnaviridae Infections/*immunology/*prevention & control/virology
Bursa of Fabricius/immunology/virology
Cell Proliferation
Chickens
CpG Islands/immunology
Enzyme-Linked Immunosorbent Assay/veterinary
Immunization/methods/*veterinary
Infectious bursal disease virus/*immunology
Interferon-gamma/immunology/therapeutic use
Lymphocytes/cytology/immunology
Oligonucleotides/immunology
Poultry Diseases/immunology/*prevention & control/*virology
Specific Pathogen-Free Organisms
Vaccines, DNA/immunology/therapeutic use
Viral Vaccines/*immunology/therapeutic use
Figure
Cited by 1 articles
-
Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-γ
Jeong Ho Park, Haan Woo Sung, Byung Il Yoon, Hyuk Moo Kwon
J Vet Sci. 2009;10(2):131-139. doi: 10.4142/jvs.2009.10.2.131.
Reference
-
1. Cao M, Sasaki O, Yamada A, Imanishi J. Enhancement of the protective effect of inactivated influenza virus vaccine by cytokines. Vaccine. 1992. 10:238–242.
Article2. Cao YC, Yeung WS, Law M, Bi YZ, Leung FC, Lim BL. Molecular characterization of seven Chinese isolates of infectious bursal disease virus: classical, very virulent, and variant strains. Avian Dis. 1998. 42:340–351.
Article3. Chang HC, Lin TL, Wu CC. DNA mediated vaccination against infectious bursal disease in chickens. Vaccine. 2001. 20:328–335.
Article4. Chettle N, Stuart JC, Wyeth PJ. Outbreak of virulent infectious bursal disease in East Anglia. Vet Rec. 1989. 125:271–272.
Article5. Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, Tao MH. Development of Th1 and Th2 populations and the nature of immune response to hepatitis B virus DNA vaccines can be modulated by co-delivery of various cytokine genes. J Immunol. 1998. 160:1320–1329.6. Digby MR, Lowenthal JW. Cloning and expression of the chicken interferon-ã gene. J Interferon Cytokine Res. 1995. 15:939–945.
Article7. Dzata GK, Confer AW, Wyckoff JH 3rd. The effects of adjuvants on immune response in cattle injected with a Brucella abortus soluble antigen. Vet Microbiol. 1991. 29:27–48.
Article8. Farrar MA, Schreiber RD. The molecular cell biology of interferon-ã and it's receptor. Annu Rev Immunol. 1993. 11:571–611.9. Fodor I, Horvath E, Fodor N, Nagy E, Rencendorsh A, Vakharia VN, Dube SK. Induction of protective immunity in chickens immunised with plasmid DNA encoding infectious bursal disease virus antigens. Acta Vet Hung. 1999. 47:481–492.
Article10. Harms JS, Oliveira SC, Splitter GA. Regulation of transgene expression in genetic immunization. Braz J Med Biol Res. 1999. 32:155–162.
Article11. Heath AW, Playfair JHL. Cytokines as immunological adjuvants. Vaccine. 1992. 10:427–434.
Article12. Hicks CR, Turner KV. Fundamental Concepts in the Design of Experiments. 1996. 5th ed. New York: Oxford University Press;195–196.13. Hilgers LA, Nicolas I, Lejeune G, Dewil E, Boon B. Effect of various adjuvants on secondary immune response in chickens. Vet Immunol Immunopathol. 1998. 66:159–171.
Article14. Hilgers LA, Snippe H. DDA as an immunological adjuvant. Res Immunol. 1992. 143:494–503.
Article15. Hofhuis FM, Van der Meer C, Kersten MCM, Rutten VPMG, Willers JMN. Effects of dimethyldioctadecylammonium bromide on phagocytosis and digestion of Listeria monocytogenes by mouse peritoneal macrophages. Immunology. 1981. 43:425–431.16. Katz D, Inbar I, Samina I, Peleg BA, Heller DE. Comparison of dimethyl dioctadecyl ammonium bromide, Freund\'s complete adjuvant and mineral oil for induction of humoral antibodies, cellular immunity and resistance to Newcastle disease virus in chickens. FEMS Immunol Med Microbiol. 1993. 7:303–313.
Article17. Kim SJ, Sung HW, Han JH, Jackwood D, Kwon HM. Protection against very virulent infectious bursal disease virus in chickens immunized with DNA vaccines. Vet Microbiol. 2004. 101:39–51.
Article18. Kraaijeveld CA, Ia Riviere G, Benaissa-Trouw BJ, Jansen J, Harmsen M, Snippe H. Effect of the adjuvant dimethyl dioctadecyl ammonium bromide on the humoral and cellular immune responses to encephalomyocarditis virus. Antiviral Res. 1983. 3:137–149.
Article19. Kraaijeveld CA, Snippe H, Harmsen M, Khader Boutahar-Trouw B. Dimethyl dioctadecylammonium-bromide as an adjuvant for delayed type hypersensitivity and cellular immunity against Semliki Forest virus in mice. Arch Virol. 1980. 65:211–217.
Article20. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995. 374:546–549.
Article21. Kwon YK, Mo IP, Seong HW, Kang MI, Koh HB, Lee JG, Yang CK. Studies on the pathogenicity of infectious bursal disease virus (SH/92) isolated in Korea. RDA J Agric Sci. 1995. 37:637–647.22. Lillehoj HS, Choi KD. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 1998. 42:307–314.
Article23. Lillehoj HS, Lindblad EB, Nichols M. Adjuvanticity of dimethyl dioctadecyl ammonium bromide, complete Freund's adjuvant and Corynebacterium parvum with respect to host immune response to coccidial antigens. Avian Dis. 1993. 37:731–740.
Article24. Lipford GB, Sparwasser T, Bauer M, Zimmermann S, Koch ES, Heeg K, Wagner H. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997. 27:3420–3426.
Article25. Lowenthal JW, O'Neil TE, Broadway M, David A, Strom G, Digby MR, Andrew M, York JJ. Coadministration of IFN-ã enhances antibody responses in chickens. J Interferon Cytokine Res. 1998. 18:617–622.
Article26. Lukert PD, Saif YM. Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. Infectious bursal disease. Diseases of Poultry. 1985. Ames: Iowa State University Press;721–738.27. Martin A, Lillehoj HS, Kaspers B, Bacon LD. Mitogen-induced lymphocyte proliferation and interferon production following coccidia infection. Avian Dis. 1994. 38:262–268.
Article28. Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ. Adjuvant effects of IL-1beta, IL-2, IL-8, IL-15, IFN-alpha, IFN-gamma TGF-beta4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine. 2001. 20:267–274.
Article29. Miyamoto T, Min W, Lillehoj HS. Lymphocyte proliferation response during Eimeria tenella infection assessed by new, reliable, nonradioactive colorimetric assay. Avian Dis. 2002. 46:10–16.
Article30. Oshop GL, Elankumaran S, Heckert RA. DNA vaccination in the avian. Vet Immunol Immunopathol. 2002. 89:1–12.
Article31. Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997. 8:2019–2029.
Article32. Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001. 11:333–340.
Article33. Rijke EO, Loeffen AHC, Lutticken D. Bizzini B, Bonmassar E, editors. The use of lipid amines as immunopotentiators for viral vaccines. Advances in Immunomodulation. 1998. Rome-Milan: Pythagota Press;433–443.34. Snyder DB, Vakharia VN, Savage PK. Naturally occurring-neutralizing monoclonal antibody escape variants define the epidemiology of infectious bursal disease viruses in the United States. Arch Virol. 1992. 127:89–101.
Article35. Song KD, Lillehoj HS, Choi KD, Zarlenga D, Han JY. Expression and functional characterization of recombinant chicken interferon-gamma. Vet Immunol Immunopathol. 1997. 58:321–333.
Article36. Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998. 28:2045–2054.
Article37. Sparwasser T, Miethke T, Lipford G, Borschert K, Hacker H, Heeg K, Wagner H. Bacterial DNA cause septic shock. Nature. 1997. 386:336–337.38. Srivastava IK, Liu MA. Gene vaccines. Ann Intern Med. 2003. 138:550–559.
Article39. Takehara K, Kobayashi K, Ruttanapumma R, Kamikawa M, Nagata T, Yokomizo Y, Nakamura M. Adjuvant effect of chicken interferon-gamma for inactivated Salmonella Enteritidis antigen. J Vet Med Sci. 2003. 65:1337–1341.
Article40. Vandenbroeck K, Nauwynck H, Vanderpooten A, Van Reeth K, Goddeeris B, Billiau A. Recombinant porcine IFN-gamma potentiates the secondary IgG and IgA responses to an inactivated suid herpesvirus-1 vaccine and reduces postchallenge weight loss and fever in pigs. J Interferon Cytokine Res. 1998. 18:739–744.
Article41. van Rooij EM, Glansbeek HL, Hilgers LA, te Lintelo EG, de Visser YE, Boersma WJ, Haagmans BL, Bianchi AT. Protective antiviral immune responses to pseudorabies virus induced by DNA vaccination using dimethyldioctadecylammonium bromide as an adjuvant. J Virol. 2002. 76:10540–15045.
Article42. Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002. 168:1659–1663.
Article43. Wang X, Jiang P, Deen S, Wu J, Liu X, Xu J. Efficacy of DNA vaccines against infectious bursal disease virus in chickens enhanced by coadministration with CpG oligodeoxynucleotide. Avian Dis. 2003. 47:1305–1312.
Article44. Weeratna R, Brazolot Millan CL, Krieg AM, Davis HL. Reduction of antigen expression from DNA vaccines by coadministered oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998. 8:351–356.
Article45. Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995. 2:129–135.
Article46. Xiang ZQ, He Z, Wang Y, Ertl HC. The effect of interferon-gamma on genetic immunization. Vaccine. 1997. 15:896–898.47. Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992. 36:983–997.
Article48. Yi AK, Klinman DM, Martin TL, Matson S, Krieg AM. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol. 1996. 157:5394–5402.49. Zar JH. Biostatistical Analysis. 1996. 3rd ed. Boston: Prentice Hall;228–231.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-gamma
- Co-Immunization of Plasmid DNA Encoding IL-12 and IL-18 with Bacillus Calmette-Guerin Vaccine against Progressive Tuberculosis
- Modulation of Humoral and Cell-Mediated Immunity Against Avian Influenza and Newcastle Disease Vaccines by Oral Administration of Salmonella enterica Serovar Typhimurium Expressing Chicken Interleukin-18
- Limited Effect of CpG ODN in Preventing Type 1 Diabetes in NOD Mice
- A Molecular Mucosal Adjuvant To Enhance Immunity Against Pneumococcal Infection In The Elderly