Yonsei Med J.
2011 Nov;52(6):1008-1015. 10.3349/ymj.2011.52.6.1008.
Co-Immunization of Plasmid DNA Encoding IL-12 and IL-18 with Bacillus Calmette-Guerin Vaccine against Progressive Tuberculosis
- Affiliations
-
- 1Department of Microbiology and Institute of Immunology and Immunological Disease, Yonsei University College of Medicine, Seoul, Korea. raycho@yuhs.ac
- 2Department of Biochemistry, College of Life Science & Engineering, Yonsei University, Seoul, Korea.
- 3Division of Molecular and Life Sciences, Postech Biotech Center, Pohang University of Science & Technology, Pohang, Korea.
- KMID: 1058823
- DOI: http://doi.org/10.3349/ymj.2011.52.6.1008
Abstract
- PURPOSE
Bacillus Calmette-Guerin (BCG) vaccine has widely been used to immunize against tuberculosis, but its protective efficacy is variable in adult pulmonary tuberculosis, while it is not efficiently protective against progressive infection of virulent Mycobacterium tuberculosis strains. In this study, the protective effects of plasmid DNA vaccine constructs encoding IL-12 or IL-18 with the BCG vaccine were evaluated against progressive infection of M. tuberculosis, using mouse aerosol challenge model.
MATERIALS AND METHODS
Plasmid DNA vaccine constructs encoding IL-12 or IL-18 were constructed and mice were immunized with the BCG vaccine or with IL-12 DNA or IL-18 DNA vaccine constructs together with the BCG vaccine.
RESULTS
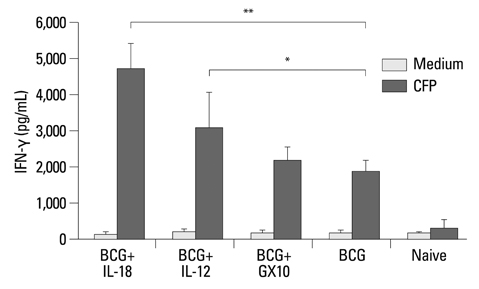
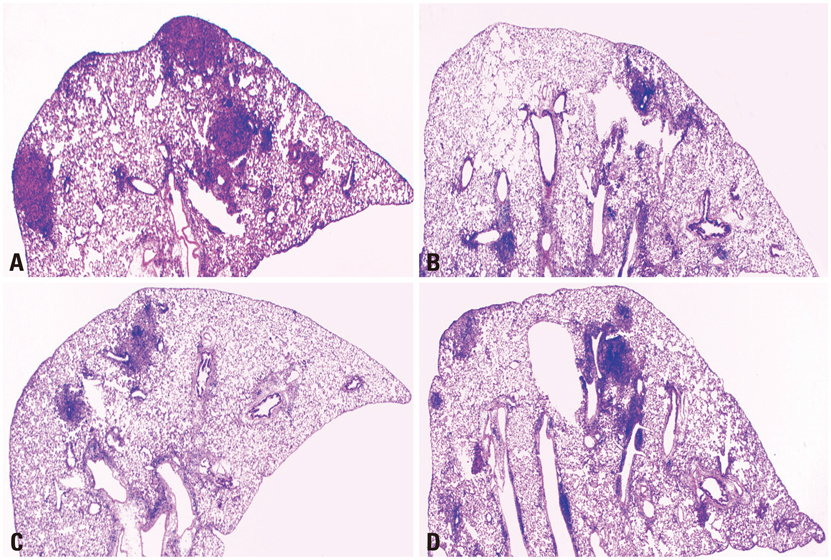
The BCG vaccine induced high level of interferon gamma (IFN-gamma) but co-immunization of IL-12 or IL-18 DNA vaccine constructs with the BCG vaccine induced significantly higher level of IFN-gamma than a single BCG vaccine. The BCG vaccine was highly protective at early stage of M. tuberculosis infection, but its protective efficacy was reduced at later stage of infection. The co-immunization of IL-12 DNA vaccine constructs with the BCG vaccine was slightly more protective at early stage of infection and was significantly more protective at later stage infection than a single BCG vaccine.
CONCLUSION
Co-immunization of IL-12 DNA vaccine with the BCG vaccine induced more protective immunity and was more effective for protection against progressive infection of M. tuberculosis.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Kinetics of IFN-Gamma and TNF-Alpha Gene Expression and Their Relationship with Disease Progression after Infection with Mycobacterium Tuberculosis in Guinea Pigs
In Soon Roh, Sungae Cho, Seok-Yong Eum, Sang-Nae Cho
Yonsei Med J. 2013;54(3):707-714. doi: 10.3349/ymj.2013.54.3.707.
Reference
-
1. Raviglione MC, Snider DE Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995. 273:220–226.
Article2. Snider DE Jr, Castro KG. The global threat of drug-resistant tuberculosis. N Engl J Med. 1998. 338:1689–1690.
Article3. Hawgood BJ. Albert Calmette (1863-1933) and Camille Guérin (1872-1961): the C and G of BCG vaccine. J Med Biogr. 2007. 15:139–146.
Article4. Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995. 346:1339–1345.
Article5. Weir RE, Gorak-Stolinska P, Floyd S, Lalor MK, Stenson S, Branson K, et al. Persistence of the immune response induced by BCG vaccination. BMC Infect Dis. 2008. 8:9.6. Derrick SC, Yang AL, Morris SL. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine. 2004. 23:780–788.
Article7. Olsen AW, Brandt L, Agger EM, van Pinxteren LA, Andersen P. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand J Immunol. 2004. 60:273–277.
Article8. Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001. 69:2773–2778.
Article9. Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest. 2005. 115:2472–2479.
Article10. Jeon BY, Derrick SC, Lim J, Kolibab K, Dheenadhayalan V, Yang AL, et al. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect Immun. 2008. 76:5173–5180.11. Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009. 50:1–11.12. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993. 178:2249–2254.
Article13. Emoto M, Emoto Y. Intracellular bacterial infection and invariant NKT cells. Yonsei Med J. 2009. 50:12–21.
Article14. Jeong E, Lee JY. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med J. 2011. 52:379–392.
Article15. Kinjo Y, Kawakami K, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Contribution of IL-18 to Th1 response and host defense against infection by Mycobacterium tuberculosis: a comparative study with IL-12p40. J Immunol. 2002. 169:323–329.
Article16. Ha SJ, Park SH, Kim HJ, Kim SC, Kang HJ, Lee EG, et al. Enhanced immunogenicity and protective efficacy with the use of interleukin-12-encapsulated microspheres plus AS01B in tuberculosis subunit vaccination. Infect Immun. 2006. 74:4954–4959.
Article17. Luo Y, Yamada H, Chen X, Ryan AA, Evanoff DP, Triccas JA, et al. Recombinant Mycobacterium bovis bacillus Calmette-Guén (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol. 2004. 137:24–34.
Article18. Martin E, Kamath AT, Briscoe H, Britton WJ. The combination of plasmid interleukin-12 with a single DNA vaccine is more effective than Mycobacterium bovis (bacille Calmette-Guérin) in protecting against systemic Mycobacterim avium infection. Immunology. 2003. 109:308–314.
Article19. Palendira U, Kamath AT, Feng CG, Martin E, Chaplin PJ, Triccas JA, et al. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect Immun. 2002. 70:1949–1956.20. Triccas JA, Sun L, Palendira U, Britton WJ. Comparative affects of plasmid-encoded interleukin 12 and interleukin 18 on the protective efficacy of DNA vaccination against Mycobacterium tuberculosis. Immunol Cell Biol. 2002. 80:346–350.
Article21. Jeon BY, Kim HJ, Kim SC, Jo EK, Park JK, Paik TH, et al. Protection of mice against Mycobacterium tuberculosis infection by immunization with aqueous fraction of Triton X-100-soluble cell wall proteins. Scand J Immunol. 2008. 67:18–23.22. Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, et al. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003. 10:1592–1599.
Article23. Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007. 117:2279–2288.
Article24. Singha H, Mallick AI, Jana C, Isore DP, Goswami TK, Srivastava SK, et al. Escheriosomes entrapped DNA vaccine co-expressing Cu-Zn superoxide dismutase and IL-18 confers protection against Brucella abortus. Microbes Infect. 2008. 10:1089–1096.
Article25. Harker JA, Godlee A, Wahlsten JL, Lee DC, Thorne LG, Sawant D, et al. Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J Virol. 2010. 84:4073–4082.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mycobacterium bovis Bacillus Calmette-Guerin (BCG) and BCG-based Vaccines Against Tuberculosis
- Efficacy of Bacillus Calmette-Guérin in Cancer Prevention and Its Putative Mechanisms
- Detection or Interleukin 2 In The Urine of Patients with Superficial Bladder Tumors after Intravesical BCG Therapy
- Generalized Granuloma Annulare in Infancy Following Bacillus Calmette-Guerin Vaccination
- Bladder Cancer Medication Bacillus Calmette-Guérin-Cell Wall Skeleton Focusing on Alternatives and Developments to Limitations