Analytical Performance Evaluation of Glucose Monitoring System Following ISO15197
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. sailchun@amc.seoul.kr
- KMID: 1077418
- DOI: http://doi.org/10.3343/kjlm.2009.29.5.423
Abstract
- BACKGROUND
We have evaluated the analytical performance of SureStep Flexx (Johnson and Johnson, USA) which can report the plasma equivalent glucose test results and be connected to the hospital information networks, following ISO15197 analytic procedure for glucometer for the first time.
METHODS
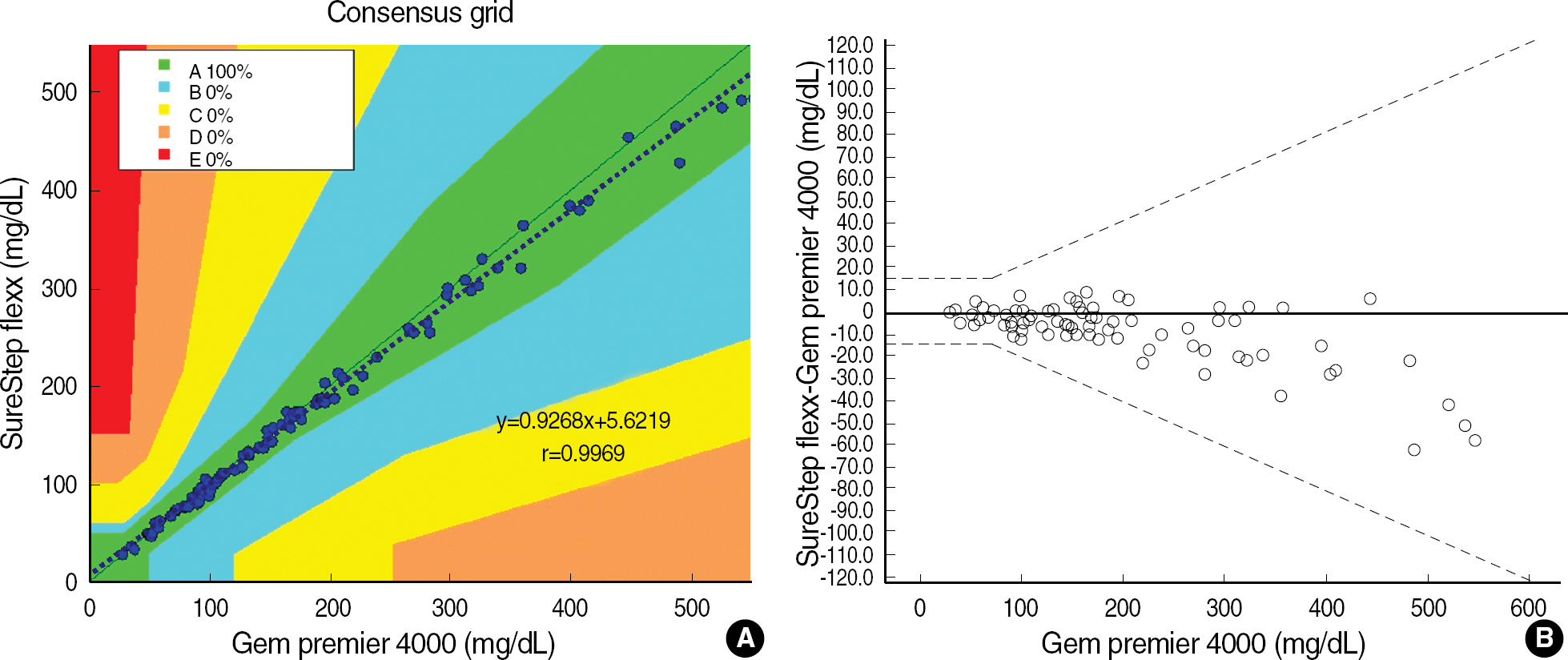
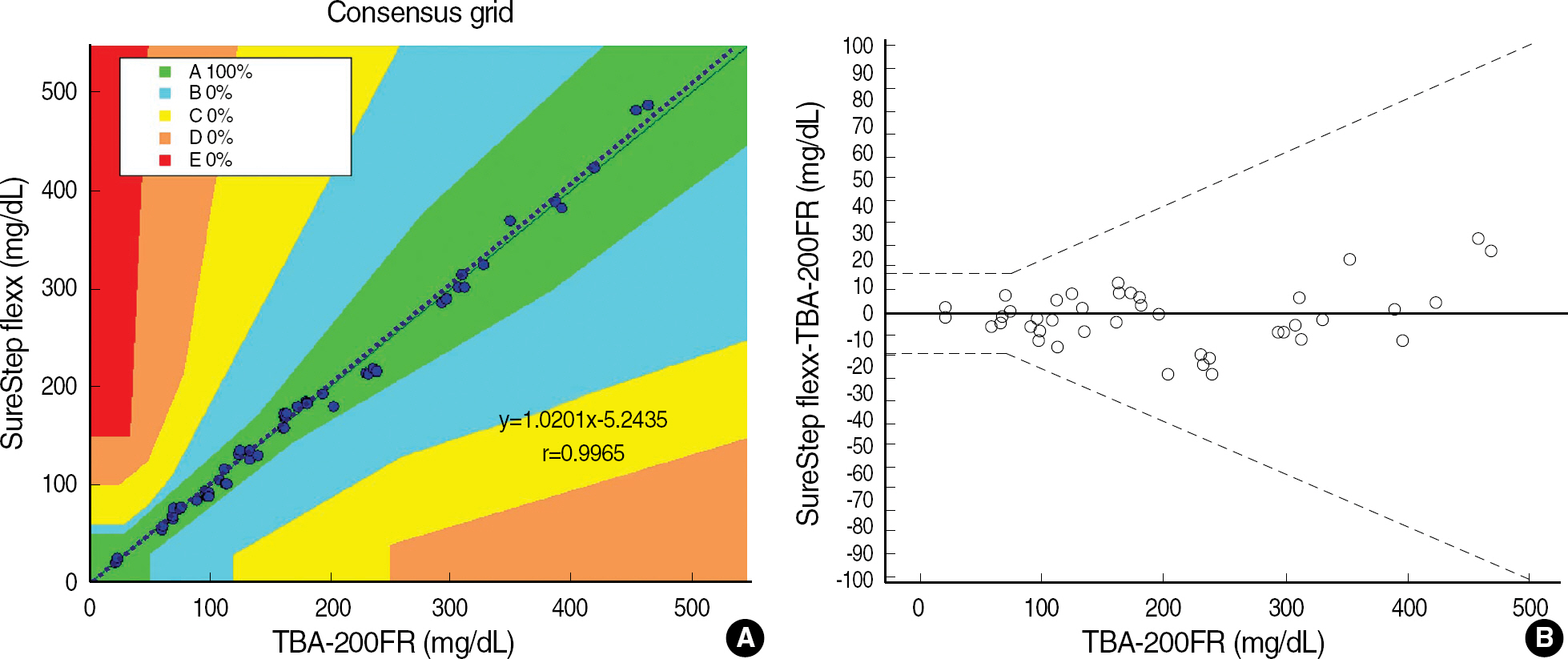
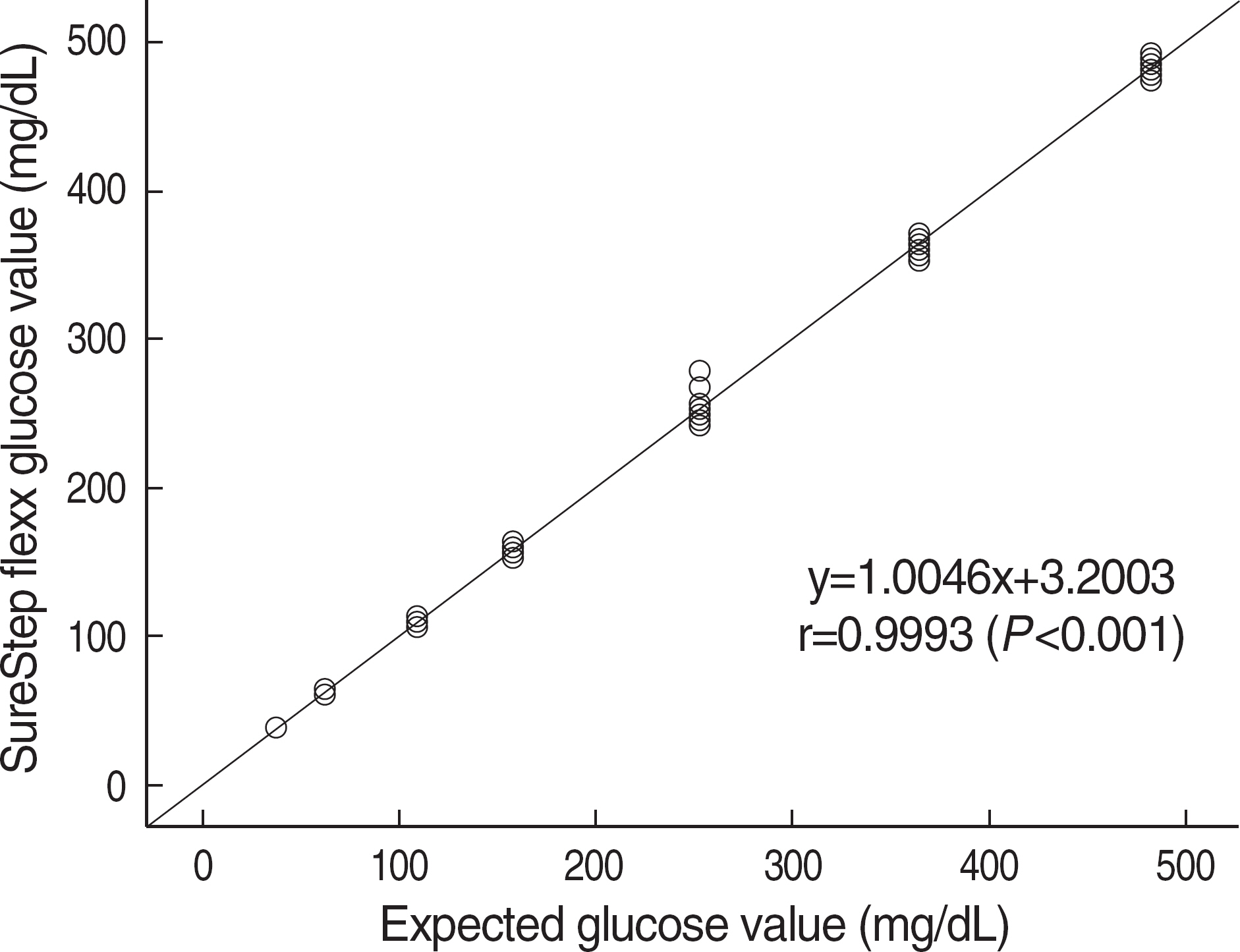
Adopting the guidelines of ISO15197, we measured the precision of ten glucometers from their repeatability and intermediate precision, and determined the accuracies of the glucometer, comparing to those of GEM Premier 4000 (Instrumentation Laboratory, USA). In addition, the guidelines of CLSI EP9-A2 and EP6-A were applied to correlate between data of glucometer and those of laboratory reference method by TBA-200FR (Toshiba Medical Systems, Japan) and to examine its linearity of glucose concentrations measured by SureStep Flexx. We used the clinical specimens and commercial control materials.
RESULTS
Repeatabilities and intermediate precisions of those glucometers were 4.0-7.3%, and 4.3-6.2%, respectively. When glucose levels are under 75 mg/dL, the difference between results of those meters and the reference values were within +/-6 mg/dL. However when glucose levels are over 75 mg/dL, those differences were within +/-12.7%. These results were acceptable for the ISO15197 criteria in all glucose concentrations. The glucose concentrations showed the clinically relevant linearity in the range from 36 mg/dL to 491 mg/dL. Moreover, Error Grid Analysis showed that all glucose results were in "zone A", which means that these values were clinically accurate.
CONCLUSIONS
This study showed that SureStep Flexx can provide reliable results for patients and clinicians to manage the diabetes mellitus, satisfying the ISO15197 criteria.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Performance Evaluation of BAROZEN H, a Networking Blood Glucose Monitoring System for Medical Institutions
Jun Hyung Lee, Young Joo Cha
Lab Med Online. 2015;5(2):69-76. doi: 10.3343/lmo.2015.5.2.69.Accuracy of Capillary Blood Glucose Test When Fasting in Diabetes Patients or General Population: Performance Evaluation of G300 Based on ISO 15197:2013 Standards
Young-min Jee, Min-hee Seo, Byung-wook Yoo, Sung-ho Hong, Choo-yon Cho, Yong-jin Cho, Jung-eun Oh, Kyung-suk Shin
Korean J Health Promot. 2017;17(4):259-268. doi: 10.15384/kjhp.2017.17.4.259.The Education Effect of Glucometer Use on the Glucose Levels and the Glucose Value Comparison among Diverse Glucometers
Kyung Ae Lee, Soyeon Jeon, Woong Ji Kim, Heung Yong Jin, Ji Hyun Park, Hong Sun Baek, Tae Sun Park
J Korean Diabetes. 2011;12(2):113-121. doi: 10.4093/jkd.2011.12.2.113.Evaluation of the Self-Testing Blood Glucose Monitoring System GlucoDr.S According to ISO 15197:2013 Guidelines
Namhee Kim, Bo Gyung Kim, Sun-Hee Jun, Kyunghoon Lee, Tae Jung Oh, Sung Hee Choi, Soo Lim, Sang Hoon Song, Woon Heung Song, Junghan Song, Hak Chul Jang
Lab Med Online. 2018;8(3):77-86. doi: 10.3343/lmo.2018.8.3.77.
Reference
-
1.Welschen LM., Bloemendal E., Nijpels G., Dekker JM., Heine RJ., Stalman WA, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005. 28:1510–7.2.The International Organization for Standardization. In vitro diagnostic test systems-requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus. ISO/TC 212/SC. International Standard ISO 15197. Geneva, Switzerland: ISO. 2003.3.Burnett RW., D'Orazio P., Fogh-Andersen N., Kuwa K., Kulpmann WR., Larsson L, et al. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001. 307:205–9.4.Beneteau-Burnat B., Pernet P., Pilon A., Latour D., Goujon S., Feuillu A, et al. Evaluation of the GEM Premier 4000: a compact blood gas CO-Oximeter and electrolyte analyzer for point-of-care and laboratory testing. Clin Chem Lab Med. 2008. 46:271–9.
Article5.Parkes JL., Slatin SL., Pardo S., Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000. 23:1143–8.
Article6.Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; Approved guideline-2nd ed. Document EP9-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2002.7.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures; A statistical approach; Approved guideline. Document EP6-A.Wayne, PA: Clinical and Laboratory Standards Institute;2003.8.Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)-HCFA. Final rule with comment period. Fed Regist. 1992. 57:7002–186.9.U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health. Draft guidance for industry and FDA staff - Total product life cycle for portable invasive blood glucose monitoring systems. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071439.pdf. (updated on Oct 2006).10.Kost GJ., Tran NK., Abad VJ., Louie RF. Evaluation of point-of-care glucose testing accuracy using locally-smoothed median absolute difference curves. Clin Chim Acta. 2008. 389:31–9.
Article11.Kilo C Sr., Dickey WT Jr., Joynes JO., Pinson MB., Baum JM., Parkes JL, et al. Evaluation of a new blood glucose monitoring system with auto-calibration for home and hospital bedside use. Diabetes Res Clin Pract. 2006. 74:66–74.
Article12.Kristensen GB., Monsen G., Skeie S., Sandberg S. Standardized evaluation of nine instruments for self-monitoring of blood glucose. Diabetes Technol Ther. 2008. 10:467–77.
Article13.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; Approved guide-line-2nd ed. Document EP5-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.14.Lee SY., Lee NY., Kim JW. Evaluation of 6 glucose testing systems. Korean J Lab Med. 2003. 23:170–9. (이수연, 이남용, 김종원. 혈당측정기6종의평가. 대한진단검사의학회지 2003;23:170-9.).15.Yoo EH., Cho HJ., Ki CS., Lee SY. Evaluation of COSMOsensor glucose monitoring system. Korean J Lab Med. 2006. 26:1–8. (유은형, 조현정, 기창석, 이수연. 자가혈당측정기 COSMOsensor 의평가. 대한진단검사의학회지 2006;26:1-8.).
Article16.Kwon MJ., Lee SY. Evaluation of GLUCOCARD X-METER glucose monitoring system. Korean J Lab Med. 2008. 28:8–15. (권민정및이수연. 혈당측정기 GLUCOCARD X-METER평가. 대한진단검사의학회지. 2008;28:8-15.).
Article17.Singh Dhatt G., Agarwal M., Bishawi B. Evaluation of a glucose meter against analytical quality specifications for hospital use. Clin Chim Acta. 2004. 343:217–21.
Article18.Clinical and Laboratory Standards Institute. Point-of-care blood glucose testing in acute and chronic care facilities; Approved guideline-2nd ed. Document C30-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2002.19.Ghys T., Goedhuys W., Spincemaille K., Gorus F., Gerlo E. Plasma-equivalent glucose at the point-of-care: evaluation of Roche Accu-Chek Inform and Abbott Precision PCx glucose meters. Clin Chim Acta. 2007. 386:63–8.
Article20.Hoedemaekers CW., Klein Gunnewiek JM., Prinsen MA., Willems JL., Van der Hoeven JG. Accuracy of bedside glucose measurement from three glucometers in critically ill patients. Crit Care Med. 2008. 36:3062–6.
Article21.Kavsak PA., Zielinski N., Li D., McNamara PJ., Adeli K. Challenges of implementing point-of-care testing (POCT) glucose meters in a pediatric acute care setting. Clin Biochem. 2004. 37:811–7.
Article22.Park AJ., Kim HR., Lee MK. Networking experience of point-of-care test glucometer. Korean J Lab Med. 2006. 26:294–8. (박애자, 김혜련,이미경. 혈당현장검사의전산망연결경험. 대한진단검사의학회지 2006;26:294-8.).
Article23.Cha CH., Lee W., Lee YW., Kim YC., Kim YH., Chun S, et al. Installation of data management system for point-of-care blood gas analyzers using local area network. Korean J Lab Med. 2005. 25:471–6. (차충 환, 이우창, 이용화, 김영철, 김윤희, 전사일 등. 근거리 통신망을 이용한 혈액가스현장검사 정보관리 시스템 구축. 대한진단검사의학회지 2005;25:471-6.).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Accuracy of Capillary Blood Glucose Test When Fasting in Diabetes Patients or General Population: Performance Evaluation of G300 Based on ISO 15197:2013 Standards
- Evaluation of GLUCOCARD X-METER Glucose Monitoring System
- Evaluation of the Self-Testing Blood Glucose Monitoring System GlucoDr.S According to ISO 15197:2013 Guidelines
- Performance Evaluation of BAROZEN H, a Networking Blood Glucose Monitoring System for Medical Institutions
- Analytic Performance Evaluation of Blood Monitoring System G400 according to ISO 15197:2013