J Vet Sci.
2006 Jun;7(2):143-150. 10.4142/jvs.2006.7.2.143.
Alteration of nitrergic neuromuscular transmission as a result of acute experimental colitis in rat
- Affiliations
-
- 1Department of Physiology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. isyang@snu.ac.kr
- 2Department of Physiology, College of Veterinary Medicine, Kyungpook National University, Daegu 702-701, Korea.
- KMID: 1059200
- DOI: http://doi.org/10.4142/jvs.2006.7.2.143
Abstract
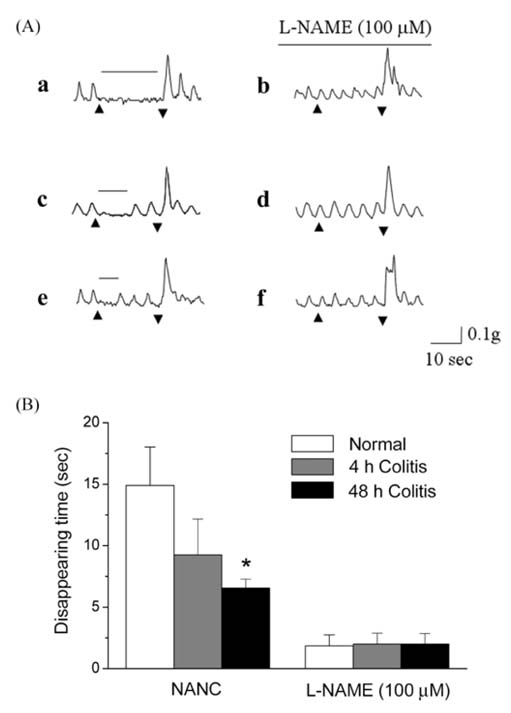
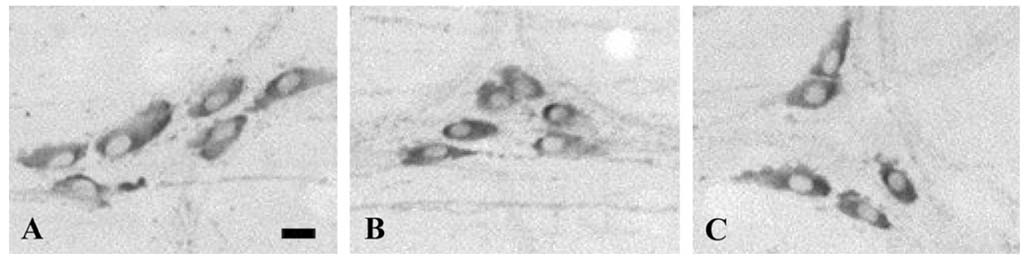
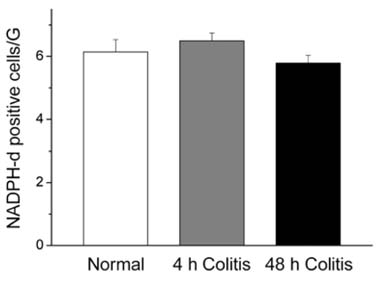
- Nitric oxide (NO) is a non-adrenergic, non-cholinergic neurotransmitter found in the enteric nervous system that plays a role in a variety of enteropathies, including inflammatory bowel disease. Alteration of nitrergic neurons has been reported to be dependent on the manner by which inflammation is caused. However, this observed alteration has not been reported with acetic acid-induced colitis. Therefore, the purpose of the current study was to investigate changes in nitrergic neuromuscular transmission in experimental colitis in a rat model. Distal colitis was induced by intracolonic administration of 4% acetic acid in the rat. Animals were sacrificed at 4 h and 48 h postacetic acid treatment. Myeloperoxidase activity was significantly increased in the acetic acid-treated groups. However, the response to 60 mM KCl was not significantly different in the three groups studied. The amplitude of phasic contractions was increased by Nomega-nitro-L-arginine methyl ester (L-NAME) in the normal control group, but not in the acetic acid-treated groups. Spontaneous contractions disappeared during electrical field stimulation (EFS) in normal group. However, for the colitis groups, these contractions initially disappeared, and then reappeared during EFS. Moreover, the observed disappearance was diminished by L-NAME; this suggests that these responses were NO-mediated. In addition, the number of NADPH-diaphorase positive nerve cell bodies, in the myenteric plexus, was not altered in the distal colon; whereas the area of NADPH-diaphorase positive fibers, in the circular muscle layer, was decreased in the acetic acidtreated groups. These results suggest that NO-mediated inhibitory neural input, to the circular muscle, was decreased in the acetic acid-treated groups.
Keyword
MeSH Terms
-
Acetic Acid/toxicity
Animals
Colitis/chemically induced/*pathology/*physiopathology
Colon/drug effects/enzymology/*innervation/pathology
Indicators and Reagents/toxicity
Male
Muscle Contraction/drug effects
Muscle, Smooth/drug effects/metabolism
Myenteric Plexus/pathology
NADPH Dehydrogenase/metabolism
NG-Nitroarginine Methyl Ester/pharmacology
Neuromuscular Junction/drug effects/*metabolism
Nitrergic Neurons/drug effects/*metabolism
Nitric Oxide/*metabolism
Peroxidase/metabolism
Potassium Chloride/pharmacology
Rats
Rats, Sprague-Dawley
Figure
Reference
-
1. Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993. 53:553–560.
Article2. Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002. 15(2 Pt 1):170–175.
Article3. Bandyopadhyay A, Chakder S, Rattan S. Regulation of inducible and neuronal nitric oxide synthase gene expression by interferon-gamma and VIP. Am J Physiol. 1997. 272(6 Pt 1):C1790–C1797.
Article4. Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997. 113:1224–1232.
Article5. Barbiers M, Timmermans JP, Scheuermann DW, Adriaensen D, Mayer B, De Groodt-Lasseel MH. Nitric oxide synthase-containing neurons in the pig large intestine: topography, morphology, and viscerofugal projections. Microsc Res Tech. 1994. 29:72–78.
Article6. Belai A, Schmidt HH, Hoyle CH, Hassall CJ, Saffrey MJ, Moss J, Forstermann U, Murad F, Burnstock G. Colocalization of nitric oxide synthase and NADPH-diaphorase in the myenteric plexus of the rat gut. Neurosci Lett. 1992. 143:60–64.
Article7. Bossone C, Hosseini JM, Pineiro-Carrero V, Shea-Donohue T. Alterations in spontaneous contractions in vitro after repeated inflammation of rat distal colon. Am J Physiol Gastrointest Liver Physiol. 2001. 280:G949–G957.
Article8. Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976. 294:47–60.
Article9. Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995. 109:1344–1367.
Article10. Fabia R, Willen R, Ar'Rajab A, Andersson R, Ahren B, Bengmark S. Acetic acid-induced colitis in the rat: a reproducible experimental model for acute ulcerative colitis. Eur Surg Res. 1992. 24:211–225.
Article11. Foxx-Orenstein AE, Grider JR. Regulation of colonic propulsion by enteric excitatory and inhibitory neurotransmitters. Am J Physiol. 1996. 271:G433–G437.
Article12. Goldhill JM, Sanders KM, Sjogren R, Shea-Donohue T. Changes in enteric neural regulation of smooth muscle in a rabbit model of small intestinal inflammation. Am J Physiol. 1995. 268(5 Pt 1):G823–G830.
Article13. Grider JR. Interplay of VIP and nitric oxide in regulation of the descending relaxation phase of peristalsis. Am J Physiol. 1993. 264(2 Pt 1):G334–G340.
Article14. Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991. 88:2811–2814.
Article15. Hosseini JM, Goldhill JM, Bossone C, Pineiro-Carrero V, Shea-Donohue T. Progressive alterations in circular smooth muscle contractility in TNBS-induced colitis in rats. Neurogastroenterol Motil. 1999. 11:347–356.
Article16. Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984. 87:1344–1350.
Article17. Miampamba M, Sharkey KA. Temporal distribution of neuronal and inducible nitric oxide synthase and nitrotyrosine during colitis in rats. Neurogastroenterol Motil. 1999. 11:193–206.
Article18. Mizuta Y, Isomoto H, Takahashi T. Impaired nitrergic innervation in rat colitis induced by dextran sulfate sodium. Gastroenterology. 2000. 118:714–723.
Article19. Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999. 277(2 Pt 1):G275–G279.
Article20. Myers BS, Martin JS, Dempsey DT, Parkman HP, Thomas RM, Ryan JP. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol. 1997. 273(4 Pt 1):G928–G936.21. Rao SS, Read NW, Holdsworth CD. Is the diarrhoea in ulcerative colitis related to impaired colonic salvage of carbohydrate? Gut. 1987. 28:1090–1094.
Article22. Rao SS, Read NW. Gastrointestinal motility in patients with ulcerative colitis. Scand J Gastroenterol Suppl. 1990. 172:22–28.
Article23. Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ Jr. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991. 101:1289–1297.
Article24. Shi XZ, Sarna SK. Impairment of Ca2+ mobilization in circular muscle cells of the inflamed colon. Am J Physiol Gastrointest Liver Physiol. 2000. 278:G234–G242.25. Smith JW, Castro GA. Relation of peroxidase activity in gut mucosa to inflammation. Am J Physiol. 1978. 234:R72–R79.
Article26. Snape WJ Jr, Matarazzo SA, Cohen S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut. 1980. 21:392–396.
Article27. Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998. 115:1504–1512.
Article28. Timmermans JP, Barbiers M, Scheuermann DW, Bogers JJ, Adriaensen D, Fekete E, Mayer B, Van Marck EA, De Groodt-Lasseel MH. Nitric oxide synthase immunoreactivity in the enteric nervous system of the developing human digestive tract. Cell Tissue Res. 1994. 275:235–245.
Article29. van Bergeijk JD, van Westreenen H, Adhien P, Zijlstra FJ. Diminished nitroprusside-induced relaxation of inflamed colonic smooth muscle in mice. Mediators Inflamm. 1998. 7:283–287.
Article30. Vermillion DL, Collins SM. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am J Physiol. 1988. 254(1 Pt 1):G124–G129.
Article31. Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992. 102:1524–1534.
Article32. Zhao A, Bossone C, Pineiro-Carrero V, Shea-Donohue T. Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J Pharmacol Exp Ther. 2001. 299:768–774.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lidocaine and Verapamil Enhances Neuromuscular Block Induced by Rocuronium
- An experimental study on the histopathological change of the mandibular joint by muscle alteration in rat
- Histological Study of Experimental Colitis Induced by Dextran Sulfate Sodium
- The Effects of Acute Hepatic Failure by Galactosamine on Mivacurium-induced Neuromuscular Blockade in the Cats
- Comparison of Cell Proliferation between Chronic Ulcerative Colitisand Acute Self-limited Colitis