Nutr Res Pract.
2015 Dec;9(6):586-591. 10.4162/nrp.2015.9.6.586.
Cytoprotective effect of rhamnetin on miconazole-induced H9c2 cell damage
- Affiliations
-
- 1Department of Medical Science, School of Medicine, Konkuk University, Seoul 143-701, Korea.
- 2Department of Pathology, College of Korean Medicine, Dongguk University, Gyeonggi-Do 410-820, Korea.
- 3Department of Diagnosis, College of Korean Medicine, Dongguk University, 32 Dongguk-ro, Ilsandong-gu, Goyang-si, Gyeonggi-Do 410-820, Korea. diapwh@dongguk.ac.kr
- KMID: 2313881
- DOI: http://doi.org/10.4162/nrp.2015.9.6.586
Abstract
- BACKGROUND/OBJECTIVES
Reactive oxygen species (ROS) formation is closely related to miconazole-induced heart dysfunction. Although rhamnetin has antioxidant effects, it remained unknown whether it can protect against miconazole-induced cardiomyocyte apoptosis. Thus, we investigated the effects of rhamnetin on miconazole-stimulated H9c2 cell apoptosis.
MATERIALS/METHODS
Cell morphology was observed by inverted microscope and cell viability was determined using a WelCount(TM) cell proliferation assay kit. Miconazole-induced ROS production was evaluated by fluorescence-activated cell sorting with 6-carboxy-2',7'-dichlorofluoroscein diacetate (H2DCF-DA) stain. Immunoblot analysis was used to determine apurinic/apyrimidinic endonuclease 1 (APE/Ref-1) and cleaved cysteine-aspartic protease (caspase) 3 expression. NADPH oxidase levels were measured using real-time polymerase chain reaction.
RESULTS
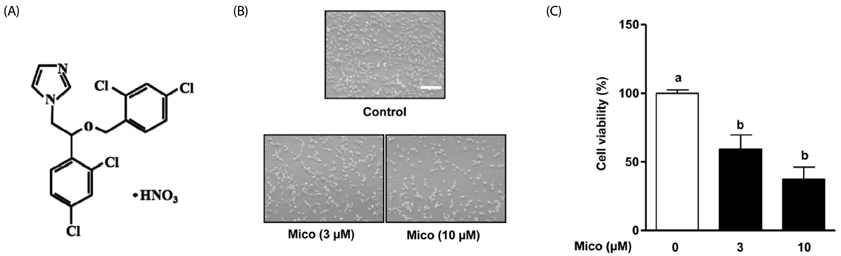
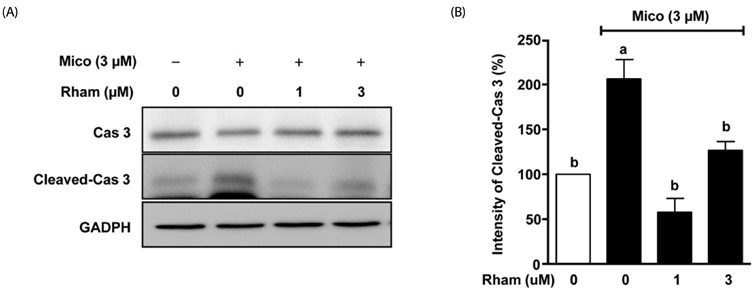
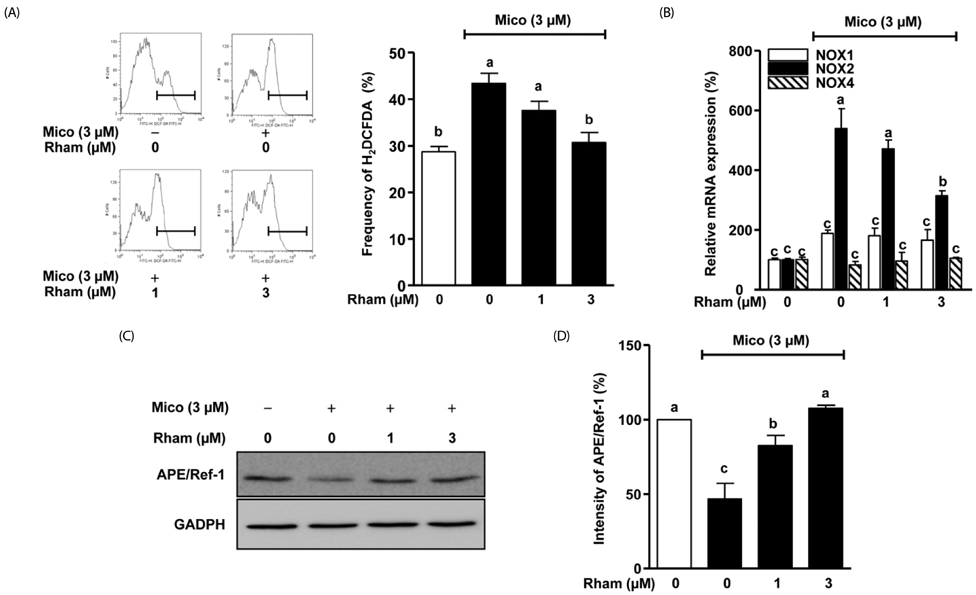
Miconazole (3 and 10 microM) induced abnormal morphological changes and cell death in H9c2 cells. Rhamnetin enhanced the viability of miconazole (3 microM)-treated cells in a dose-dependent manner. Rhamnetin (1 and 3 microM) treatment downregulated cleaved caspase 3 and upregulated APE/Ref-1 expression in miconazole-stimulated cells. Additionally, rhamnetin significantly reduced ROS generation.
CONCLUSIONS
Our data suggest that rhamnetin may have cytoprotective effects in miconazole-stimulated H9c2 cardiomyocytes via ROS inhibition. This effect most likely occurs through the upregulation of APE/Ref-1 and attenuation of hydrogen peroxide levels.
Keyword
MeSH Terms
-
Antioxidants
Apoptosis
Caspase 3
Cell Death
Cell Proliferation
Cell Survival
Flow Cytometry
Heart
Hydrogen Peroxide
Miconazole
Myocytes, Cardiac
NADPH Oxidase
Reactive Oxygen Species
Real-Time Polymerase Chain Reaction
Up-Regulation
Antioxidants
Caspase 3
Hydrogen Peroxide
Miconazole
NADPH Oxidase
Reactive Oxygen Species
Figure
Cited by 1 articles
-
Anti-neuroinflammatory effects of ethanolic extract of black chokeberry (Aronia melanocapa L.) in lipopolysaccharide-stimulated BV2 cells and ICR mice
Kang Pa Lee, Nan Hee Choi, Hyun-Soo Kim, Sanghyun Ahn, In-Sik Park, Dea Won Lee
Nutr Res Pract. 2018;12(1):13-19. doi: 10.4162/nrp.2018.12.1.13.
Reference
-
1. Mendis S, Lindholm LH, Anderson SG, Alwan A, Koju R, Onwubere BJ, Kayani AM, Abeysinghe N, Duneas A, Tabagari S, Fan W, Sarraf-Zadegan N, Nordet P, Whitworth J, Heagerty A. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J Clin Epidemiol. 2011; 64:1451–1462.
Article2. Ministry of Health and Welfare. Korea Centers for Disease Control and Prevention. Korea Health Statistics 2012: Korea National Health and Nutrition Examination Survey (KNHANES V-3). Cheongwon: Korea Centers for Disease Control and Prevention;2013.3. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993; 362:801–809.
Article4. Müller C. New ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Swiss Med Wkly. 2012; 142:w13514.
Article5. Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006; 70:181–190.
Article6. Coley KC, Crain JL. Miconazole-induced fatal dysrhythmia. Pharmacotherapy. 1997; 17:379–382.7. Kobayashi D, Kondo K, Uehara N, Otokozawa S, Tsuji N, Yagihashi A, Watanabe N. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob Agents Chemother. 2002; 46:3113–3117.
Article8. Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997; 275:1649–1652.
Article9. Dumaine R, Roy ML, Brown AM. Blockade of HERG and Kv1.5 by ketoconazole. J Pharmacol Exp Ther. 1998; 286:727–735.10. Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009; 14:536–548.
Article11. Pulkki KJ. Cytokines and cardiomyocyte death. Ann Med. 1997; 29:339–343.
Article12. Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007; 55:9427–9435.
Article13. Kim DE, Kim B, Shin HS, Kwon HJ, Park ES. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp Cell Res. 2014; 327:264–275.
Article14. Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005; 38:47–62.
Article15. Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol. 2014; 92:90–101.
Article16. Núñez-Córdoba JM, Martínez-González MA. Antioxidant vitamins and cardiovascular disease. Curr Top Med Chem. 2011; 11:1861–1869.
Article17. Won KJ, Lin HY, Jung S, Cho SM, Shin HC, Bae YM, Lee SH, Kim HJ, Jeon BH, Kim B. Antifungal miconazole induces cardiotoxicity via inhibition of APE/Ref-1-related pathway in rat neonatal cardiomyocytes. Toxicol Sci. 2012; 126:298–305.
Article18. Ozipek M, Caliş I, Ertan M, Rüedi P. Rhamnetin 3-p-coumaroylrhamninoside from Rhamnus petiolaris. Phytochemistry. 1994; 37:249–253.
Article19. Igarashi K, Ohmuma M. Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver antioxidative enzyme activities of rats. Biosci Biotechnol Biochem. 1995; 59:595–601.
Article20. Yamamoto N, Moon JH, Tsushida T, Nagao A, Terao J. Inhibitory effect of quercetin metabolites and their related derivatives on copper ion-induced lipid peroxidation in human low-density lipoprotein. Arch Biochem Biophys. 1999; 372:347–354.
Article21. Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014; 30:513–523.
Article22. Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014; 2:17.
Article23. Paneni F, Costantino S, Cosentino F. Role of oxidative stress in endothelial insulin resistance. World J Diabetes. 2015; 6:326–332.
Article24. Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015; 116:531–549.
Article25. Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res. 2010; 342:325–339.
Article26. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007; 87:245–313.
Article27. Puca R, Nardinocchi L, Starace G, Rechavi G, Sacchi A, Givol D, D'Orazi G. Nox1 is involved in p53 deacetylation and suppression of its transcriptional activity and apoptosis. Free Radic Biol Med. 2010; 48:1338–1346.
Article28. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010; 107:15565–15570.
Article29. Kuroda J, Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010; 3:314–320.
Article30. Matsuno K, Iwata K, Matsumoto M, Katsuyama M, Cui W, Murata A, Nakamura H, Ibi M, Ikami K, Zhang J, Matoba S, Jin D, Takai S, Matsubara H, Matsuda N, Yabe-Nishimura C. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radic Biol Med. 2012; 53:1718–1728.
Article31. Park ES, Kang JC, Jang YC, Park JS, Jang SY, Kim DE, Kim B, Shin HS. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J Ethnopharmacol. 2014; 153:552–560.
Article32. Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK. APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med. 2014; 46:e106.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Luteolin Protects Cardiomyocytes Cells against Lipopolysaccharide-Induced Apoptosis and Inflammatory Damage by Modulating Nlrp3
- Acetaminophen Induced Cytotoxicity and Altered Gene Expression in Cultured Cardiomyocytes of H9C2 Cells
- The Role of Sek1 in Protecting NO-induced Apoptosis in H9c2 cells by Inhibiting Caspase 3 Activation
- Adriamycin Induced Apoptosis of H9c2 Cardiomyocytes via a Caspase-Independent Pathway
- Cytoprotective effect of polysaccharide isolated from different mushrooms against 7-ketocholesterol induced damage in mouse liver cell line (BNL CL. 2)