Korean J Radiol.
2011 Feb;12(1):78-88. 10.3348/kjr.2011.12.1.78.
Evaluation of the Chondromalacia Patella Using a Microscopy Coil: Comparison of the Two-Dimensional Fast Spin Echo Techniques and the Three-Dimensional Fast Field Echo Techniques
- Affiliations
-
- 1Department of Radiology and the Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea. shlee@amc.seoul.kr
- 2Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea.
- 3Department of Orthopedic Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul 138-736, Korea.
- KMID: 991685
- DOI: http://doi.org/10.3348/kjr.2011.12.1.78
Abstract
OBJECTIVE
We wanted to compare the two-dimensional (2D) fast spin echo (FSE) techniques and the three-dimensional (3D) fast field echo techniques for the evaluation of the chondromalacia patella using a microscopy coil.
MATERIALS AND METHODS
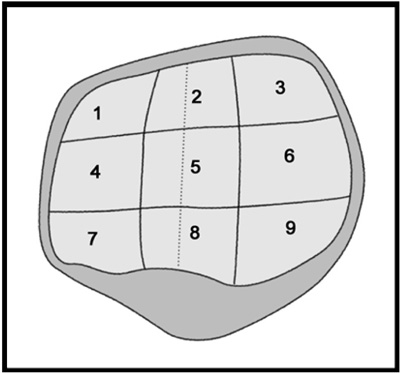
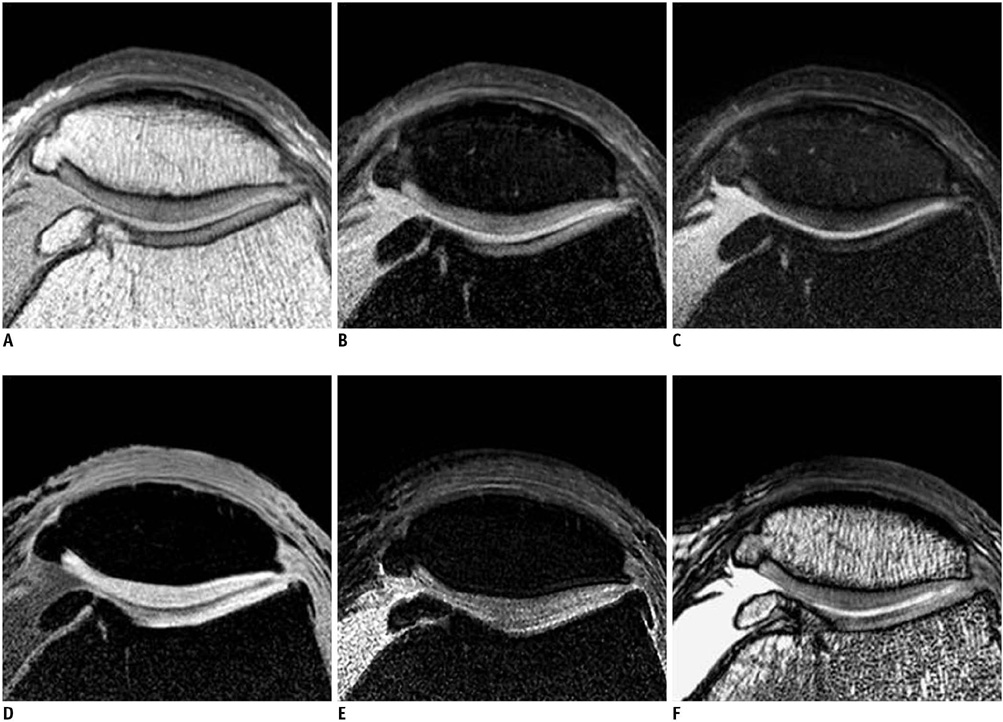
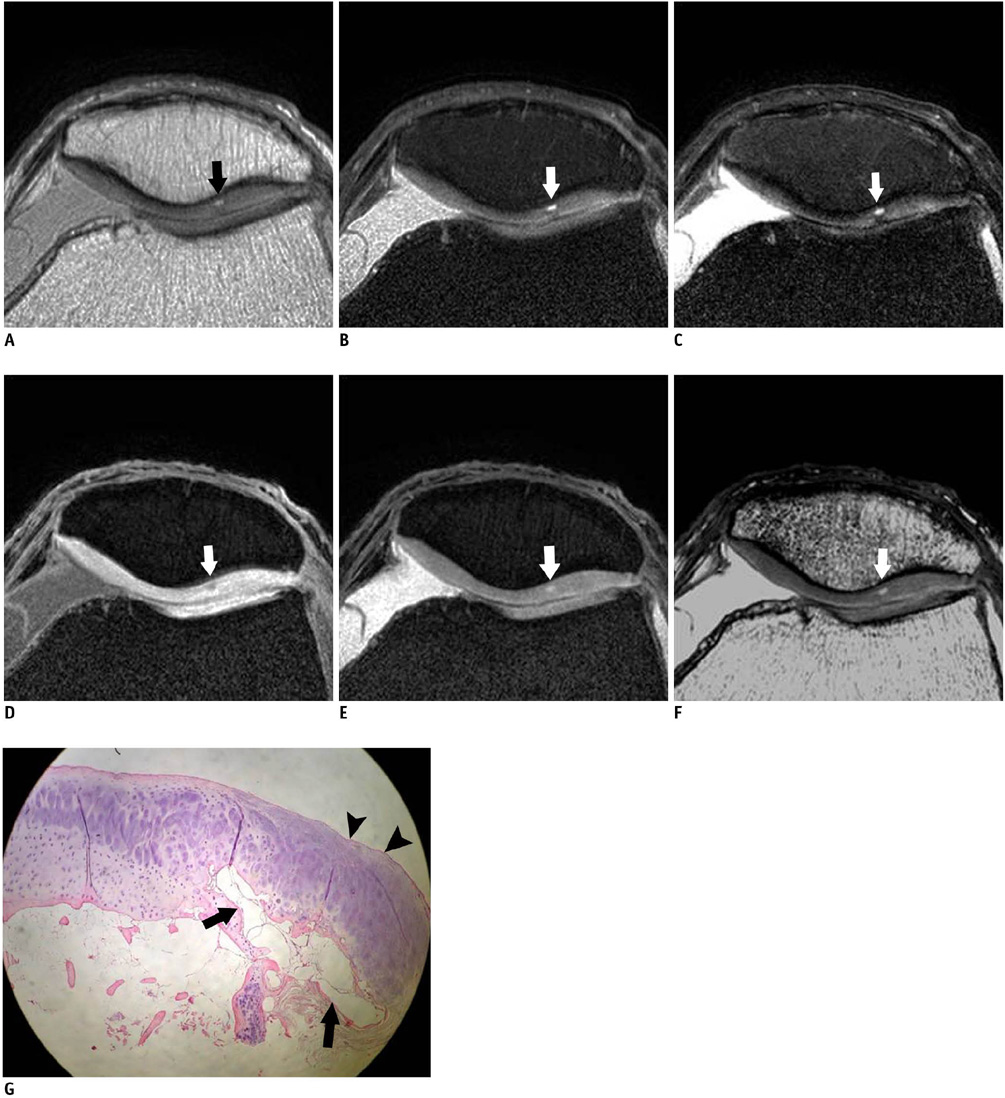
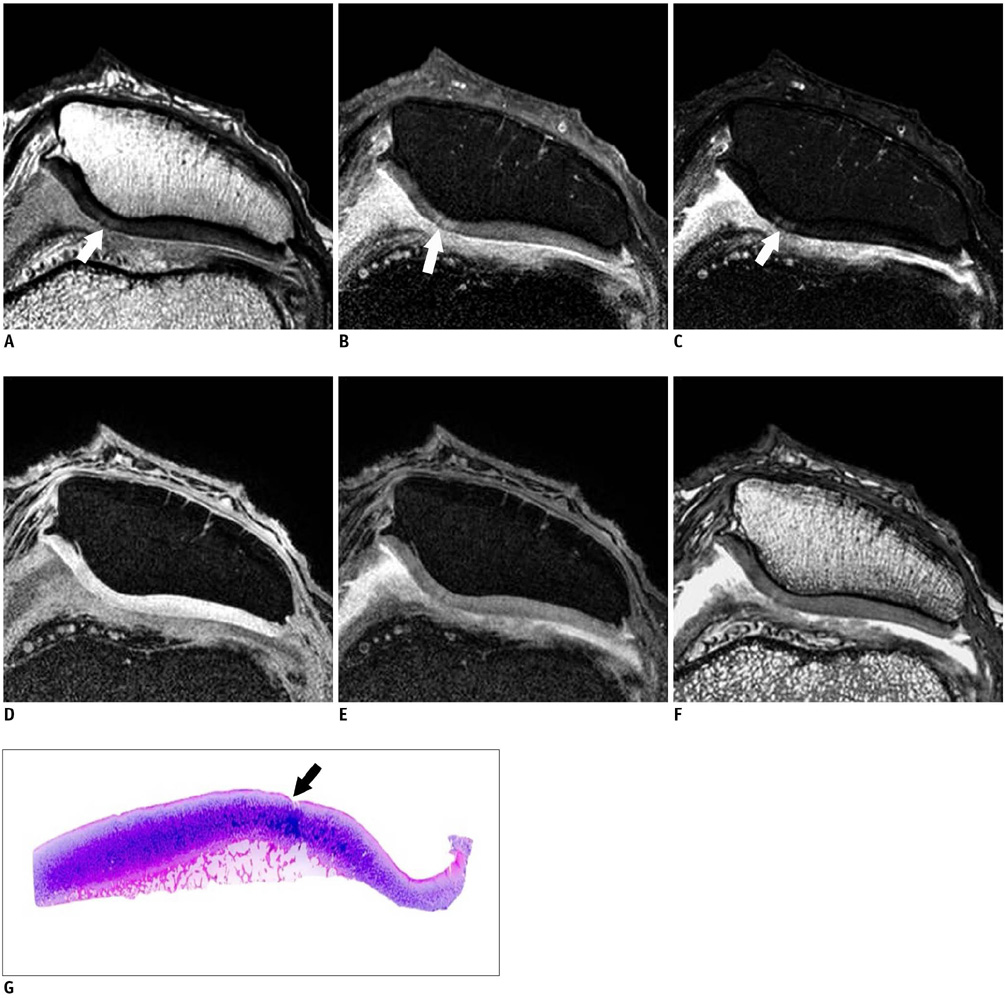
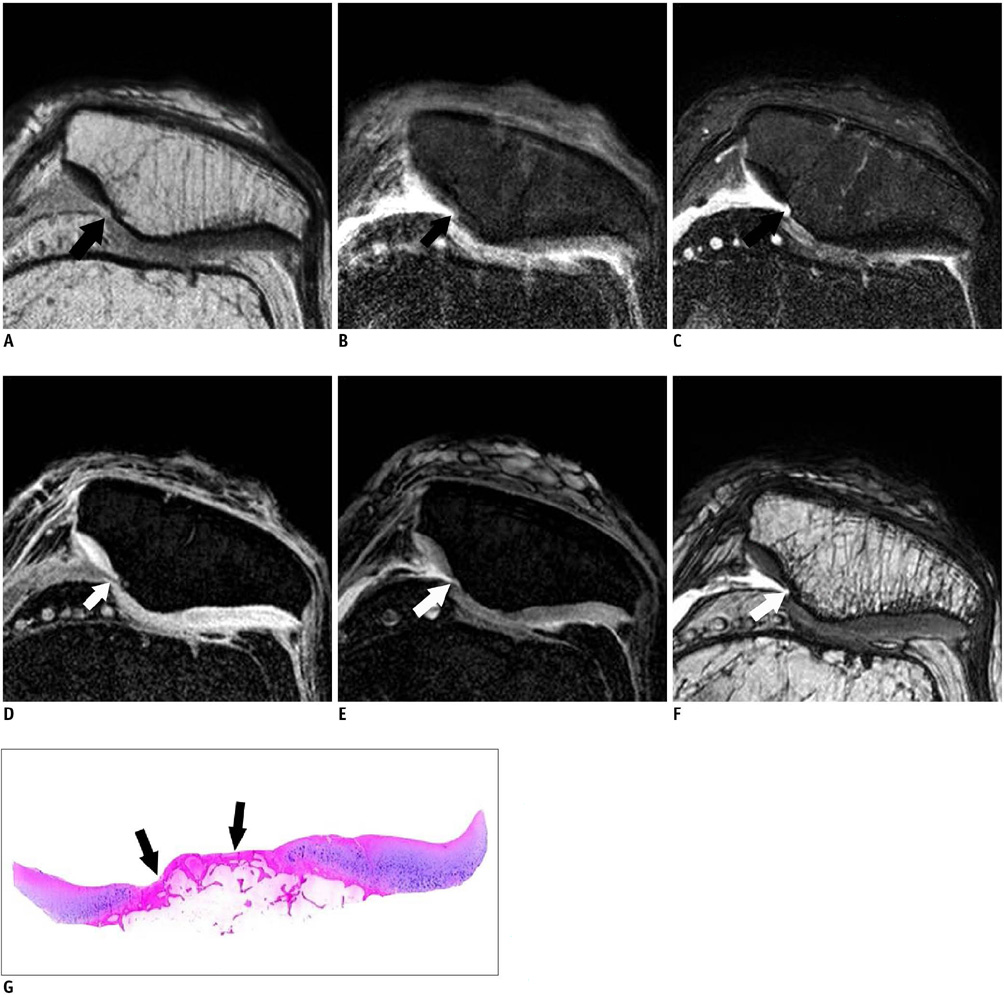
Twenty five patients who underwent total knee arthroplasty were included in this study. Preoperative MRI evaluation of the patella was performed using a microscopy coil (47 mm). The proton density-weighted fast spin echo images (PD), the fat-suppressed PD images (FS-PD), the intermediate weighted-fat suppressed fast spin echo images (iw-FS-FSE), the 3D balanced-fast field echo images (B-FFE), the 3D water selective cartilage scan (WATS-c) and the 3D water selective fluid scan (WATS-f) were obtained on a 1.5T MRI scanner. The patellar cartilage was evaluated in nine areas: the superior, middle and the inferior portions that were subdivided into the medial, central and lateral facets in a total of 215 areas. Employing the Noyes grading system, the MRI grade 0-I, II and III lesions were compared using the gross and microscopic findings. The sensitivity, specificity and accuracy were evaluated for each sequence. The significance of the differences for the individual sequences was calculated using the McNemar test.
RESULTS
The gross and microscopic findings demonstrated 167 grade 0-I lesions, 40 grade II lesions and eight grade III lesions. Iw-FS-FSE had the highest accuracy (sensitivity/specificity/accuracy = 88%/98%/96%), followed by FS-PD (78%/98%/93%, respectively), PD (76%/98%/93%, respectively), B-FFE (71%/100%/93%, respectively), WATS-c (67%/100%/92%, respectively) and WATS-f (58%/99%/89%, respectively). There were statistically significant differences for the iw-FS-FSE and WATS-f and for the PD-FS and WATS-f (p < 0.01).
CONCLUSION
The iw-FS-FSE images obtained with a microscopy coil show best diagnostic performance among the 2D and 3D GRE images for evaluating the chondromalacia patella.
MeSH Terms
Figure
Reference
-
1. Disler DG, McCauley TR, Wirth CR, Fuchs MD. Detection of knee hyaline cartilage defects using fat-suppressed three-dimensional spoiled gradient-echo MR imaging: comparison with standard MR imaging and correlation with arthroscopy. AJR Am J Roentgenol. 1995. 165:377–382.2. Mohr A, Priebe M, Taouli B, Grimm J, Heller M, Brossmann J. Selective water excitation for faster MR imaging of articular cartilage defects: initial clinical results. Eur Radiol. 2003. 13:686–689.3. Hauger O, Dumont E, Chateil JF, Moinard M, Diard F. Water excitation as an alternative to fat saturation in MR imaging: preliminary results in musculoskeletal imaging. Radiology. 2002. 224:657–663.4. Gold GE, Fuller SE, Hargreaves BA, Stevens KJ, Beaulieu CF. Driven equilibrium magnetic resonance imaging of articular cartilage: initial clinical experience. J Magn Reson Imaging. 2005. 21:476–481.5. Kornaat PR, Doornbos J, van der Molen AJ, Kloppenburg M, Nelissen RG, Hogendoorn PC, et al. Magnetic resonance imaging of knee cartilage using a water selective balanced steady-state free precession sequence. J Magn Reson Imaging. 2004. 20:850–856.6. Ruehm S, Zanetti M, Romero J, Hodler J. MRI of patellar articular cartilage: evaluation of an optimized gradient echo sequence (3D-DESS). J Magn Reson Imaging. 1998. 8:1246–1251.7. Saadat E, Jobke B, Chu B, Lu Y, Cheng J, Li X, et al. Diagnostic performance of in vivo 3-T MRI for articular cartilage abnormalities in human osteoarthritic knees using histology as standard of reference. Eur Radiol. 2008. 18:2292–2302.8. Schaefer FK, Kurz B, Schaefer PJ, Fuerst M, Hedderich J, Graessner J, et al. Accuracy and precision in the detection of articular cartilage lesions using magnetic resonance imaging at 1.5 Tesla in an in vitro study with orthopedic and histopathologic correlation. Acta Radiol. 2007. 48:1131–1137.9. Bauer JS, Barr C, Henning TD, Malfair D, Ma CB, Steinbach L, et al. Magnetic resonance imaging of the ankle at 3.0 Tesla and 1.5 Tesla in human cadaver specimens with artificially created lesions of cartilage and ligaments. Invest Radiol. 2008. 43:604–611.10. Iwama Y, Fujii M, Shibanuma H, Muratsu H, Kurosaka M, Kawamitsu H, et al. High-resolution MRI using a microscopy coil for the diagnosis of recurrent lateral patellar dislocation. Radiat Med. 2006. 24:327–334.11. Yoshioka H, Tanaka T, Ueno T, Shindo M, Carrino JA, Lang P, et al. High-resolution MR imaging of the proximal zone of the lunotriquetral ligament with a microscopy coil. Skeletal Radiol. 2006. 35:288–294.12. Hardy PA, Recht MP, Piraino DW. Fat suppressed MRI of articular cartilage with a spatial-spectral excitation pulse. J Magn Reson Imaging. 1998. 8:1279–1287.13. Recht MP, Piraino DW, Paletta GA, Schils JP, Belhobek GH. Accuracy of fat-suppressed three-dimensional spoiled gradient-echo FLASH MR imaging in the detection of patellofemoral articular cartilage abnormalities. Radiology. 1996. 198:209–212.14. Sonin AH, Pensy RA, Mulligan ME, Hatem S. Grading articular cartilage of the knee using fast spin-echo proton density-weighted MR imaging without fat suppression. AJR Am J Roentgenol. 2002. 179:1159–1166.15. Mohr A, Roemer FW, Genant HK, Liess C. Using fat-saturated proton density-weighted MR imaging to evaluate articular cartilage. AJR Am J Roentgenol. 2003. 181:280–281.16. Yoshioka H, Stevens K, Hargreaves BA, Steines D, Genovese M, Dillingham MF, et al. Magnetic resonance imaging of articular cartilage of the knee: comparison between fat-suppressed three-dimensional SPGR imaging, fat-suppressed FSE imaging, and fat-suppressed three-dimensional DEFT imaging, and correlation with arthroscopy. J Magn Reson Imaging. 2004. 20:857–864.17. Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007. 17:1135–1146.18. Disler DG. Fat-suppressed three-dimensional spoiled gradient-recalled MR imaging: assessment of articular and physeal hyaline cartilage. AJR Am J Roentgenol. 1997. 169:1117–1123.19. Peterfy CG, van Dijke CF, Janzen DL, Glüer CC, Namba R, Majumdar S, et al. Quantification of articular cartilage in the knee with pulsed saturation transfer subtraction and fat-suppressed MR imaging: optimization and validation. Radiology. 1994. 192:485–491.20. Zur Y. Design of improved spectral-spatial pulses for routine clinical use. Magn Reson Med. 2000. 43:410–420.21. Mohr A. The value of water-excitation 3D FLASH and fat-saturated PDw TSE MR imaging for detecting and grading articular cartilage lesions of the knee. Skeletal Radiol. 2003. 32:396–402.22. Yoshioka H, Stevens K, Genovese M, Dillingham MF, Lang P. Articular cartilage of knee: normal patterns at MR imaging that mimic disease in healthy subjects and patients with osteoarthritis. Radiology. 2004. 231:31–38.23. Hargreaves BA, Gold GE, Beaulieu CF, Vasanawala SS, Nishimura DG, Pauly JM. Comparison of new sequences for high-resolution cartilage imaging. Magn Reson Med. 2003. 49:700–709.24. Scheffler K. Fast frequency mapping with balanced SSFP: theory and application to proton-resonance frequency shift thermometry. Magn Reson Med. 2004. 51:1205–1211.25. Vlaardingerbroek MT, den Boer JA. Magnetic resonance imaging: theory and practice. 2003. 3rd ed. New York: Springer.26. Duc SR, Pfirrmann CW, Schmid MR, Zanetti M, Koch PP, Kalberer F, et al. Articular cartilage defects detected with 3D water-excitation true FISP: prospective comparison with sequences commonly used for knee imaging. Radiology. 2007. 245:216–223.27. Barr C, Bauer JS, Malfair D, Ma B, Henning TD, Steinbach L, et al. MR imaging of the ankle at 3 Tesla and 1.5 Tesla: protocol optimization and application to cartilage, ligament and tendon pathology in cadaver specimens. Eur Radiol. 2007. 17:1518–1528.28. Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005. 234:245–249.29. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004. 8:355–368.30. Yao L, Gentili A, Thomas A. Incidental magnetization transfer contrast in fast spin-echo imaging of cartilage. J Magn Reson Imaging. 1996. 6:180–184.31. Prock T, Collins D, Leach MO. Numerical evaluation of shaped surface coil sensitivity at 63 MHz. Phys Med Biol. 2001. 46:1753–1765.32. Gensanne D, Josse G, Lagarde JM, Vincensini D. High spatial resolution quantitative MR images: an experimental study of dedicated surface coils. Phys Med Biol. 2006. 51:2843–2855.33. Hurson C, Kashir A, Flavin R, Kelly I. Routine patellar resurfacing using an inset patellar technique. Int Orthop. 2010. 34:955–958.34. Hantes ME, Zachos VC, Bargiotas KA, Basdekis GK, Karantanas AH, Malizos KN. Patellar tendon length after anterior cruciate ligament reconstruction: a comparative magnetic resonance imaging study between patellar and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2007. 15:712–719.35. Niitsu M, Ikeda K. Magnetic resonance microscopic images with 50-mm field-of-view of the medial aspect of the knee. Acta Radiol. 2004. 45:760–768.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Meniscal Tears of the Knee: Diagnosis with Fast Spin-Echo MR Imaging and Role of Gadolinium-Enhancement
- Single-Slab 3D Fast Spin Echo and Its Variants With Very Long Echo Trains for Clinical T 2 -Weighted Contrast
- Diagnosis of Meniscal Tear of the Knee Using Proton-weighted Fast Spin-Echo MR Imaging: Can be an Alternative to Conventional Spin-Echo Imaging?
- Evaluation of Chondromalacia in the Knee Joint using Three Dimensional Fourier Transformation Constructive Interference in Steady State(CISS)
- Single-Slab 3D Fast Spin Echo Imaging: T1 -Contrast Perspective