Korean J Ophthalmol.
2010 Jun;24(3):163-168. 10.3341/kjo.2010.24.3.163.
The Anti-angiogenic Effect of Chlorogenic Acid on Choroidal Neovascularization
- Affiliations
-
- 1Department of Ophthalmology, Incheon Medical Center, Incheon, Korea.
- 2Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea.
- 3HanGil Eye Hospital, Incheon, Korea. jhsohn19@hanafos.com
- KMID: 945987
- DOI: http://doi.org/10.3341/kjo.2010.24.3.163
Abstract
- PURPOSE
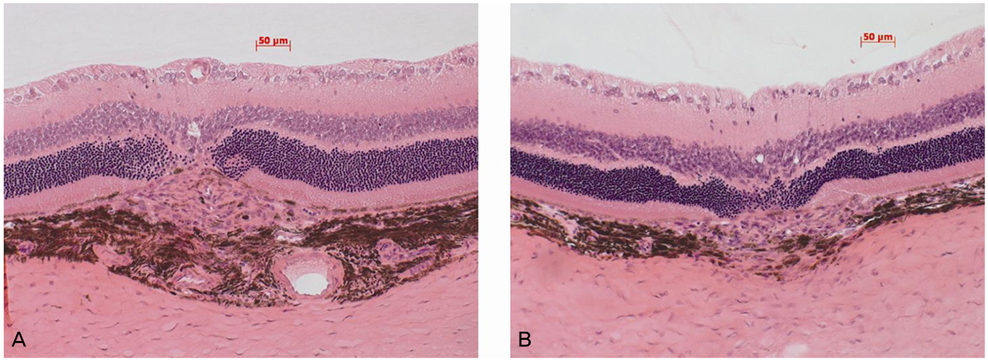
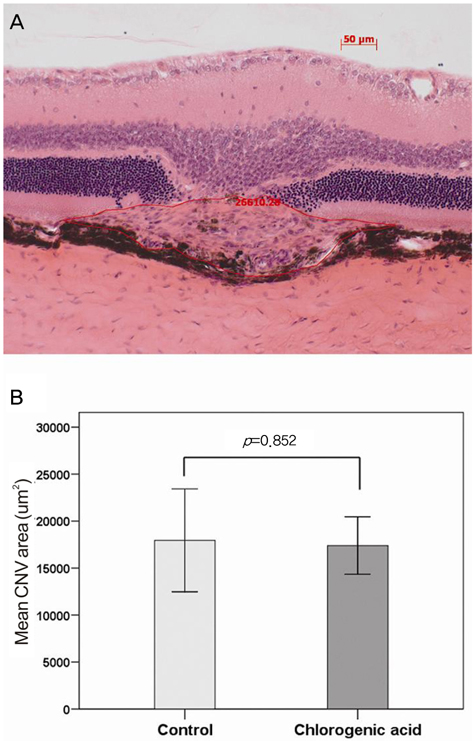
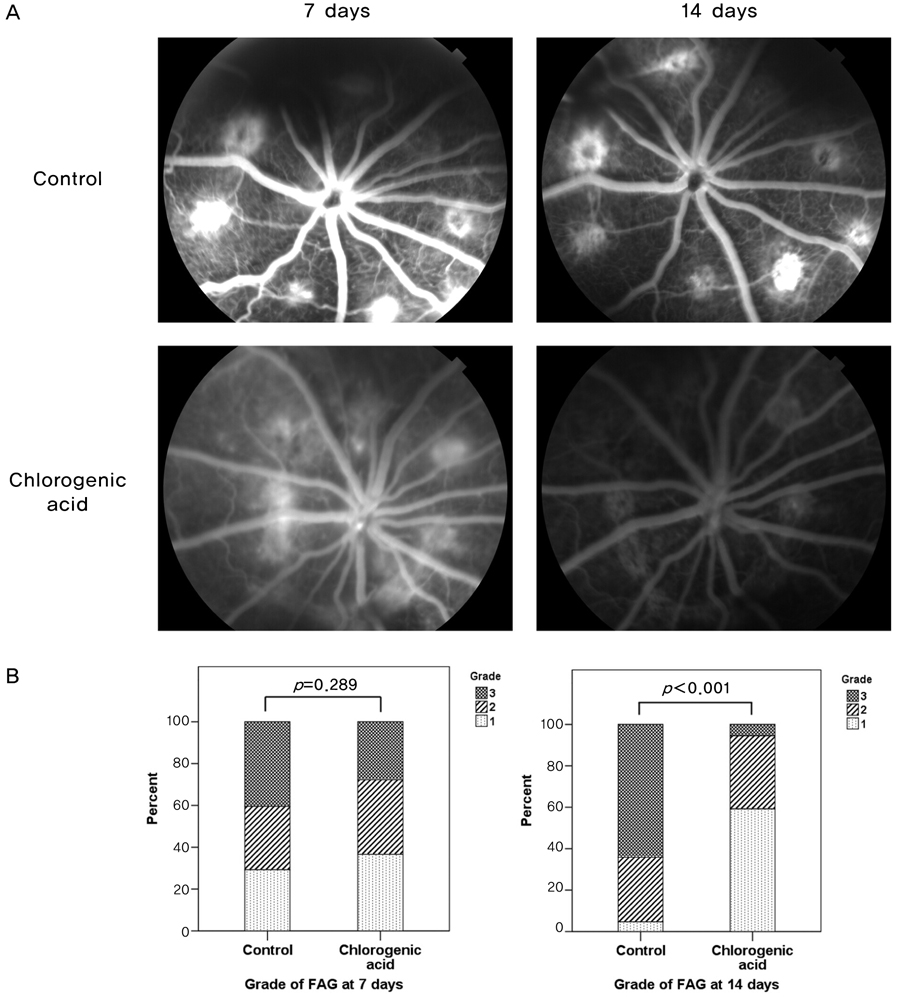
To evaluate the inhibitory effect of chlorogenic acid on laser-induced choroidal neovascularization (CNV) in a rat model. METHODS: Intraperitoneal injection of chlorogenic acid (10 mg/kg) was inititated one day prior to laser photocoagulation and continued for eight days. Eyes were removed 14 days after laser photocoagulation. Fluorescein angiography was employed at seven and 14 days to assess the CNV lesions, and histological examination was performed. Quantification of CNV size and leakage were performed both in histological sections and fluorescein angiography in order to compare the inhibitory effects of chlorogenic acid on CNV with the results of the control. RESULTS: Histological analysis showed no significant difference in CNV size between the treated and control groups. However, CNV leakage on fluorescein angiography had significantly decreased in the chlorogenic acid-treated group at 14 days after laser photocoagulation compared with that of the control group. In addition, CNV size on fluorescein angiography had significantly decreased in the treated group at seven and 14 days. CONCLUSIONS: These results suggest that chlorogenic acid has anti-angiogenic effects on CNV and may be useful as an inhibitor in the treatment or prevention of neovascular age-related macular degeneration.
MeSH Terms
-
Angiogenesis Inhibitors/*administration & dosage
Animals
Capillary Permeability/drug effects
Chlorogenic Acid/*administration & dosage
Choroid/pathology
Choroidal Neovascularization/diagnosis/etiology/*physiopathology
Fluorescein Angiography
Injections, Intraperitoneal
Laser Coagulation
Radiation Injuries
Rats
Rats, Inbred BN
Figure
Reference
-
1. Folkman J. Angiogenesis. Annu Rev Med. 2006. 57:1–18.2. Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995. 36:182–191.3. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005. 25:111–118.4. Clifford MN, Johnston KL, Knight S, Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem. 2003. 51:2900–2911.5. Yen WJ, Wang BS, Chang LW, Duh PD. Antioxidant properties of roasted coffee residues. J Agric Food Chem. 2005. 53:2658–2663.6. Krakauer T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol Immunotoxicol. 2002. 24:113–119.7. Lambert JD, Hong J, Yang GY, et al. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005. 81:284S–291S.8. Kandaswami C, Lee LT, Lee PP, et al. The antitumor activities of flavonoids. In Vivo. 2005. 19:895–909.9. Kim DO, Heo HJ, Kim YJ, et al. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 2005. 53:9921–9927.10. Paynter NP, Yeh HC, Voutilainen S, et al. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006. 164:1075–1084.11. Morton LW, Abu-Amsha Caccetta R, Puddey IB, Croft KD. Chemistry and biological effects of dietary phenolic compounds: relevance to cardiovascular disease. Clin Exp Pharmacol Physiol. 2000. 27:152–159.12. Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005. 81:317S–325S.13. Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003. 17:2290–2292.14. Schmidt-Erfurth U. Nutrition and retina. Dev Ophthalmol. 2005. 38:120–147.15. Padda MS, Picha DH. Methodology optimization for quantification of total phenolics and individual phenolic acids in sweetpotato (Ipomoea batatas L.) roots. J Food Sci. 2007. 72:C412–C416.16. Chinnici F, Bendini A, Gaiani A, Riponi C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J Agric Food Chem. 2004. 52:4684–4689.17. Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond). 2007. 4:8.18. Mrudula T, Suryanarayana P, Srinivas PN, Reddy GB. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem Biophys Res Commun. 2007. 361:528–532.19. Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000. 21:505–515.20. Carmeliet P. Developmental biology. Controlling the cellular brakes. Nature. 1999. 401:657–658.21. Das A, McGuire PG, Eriqat C, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999. 40:809–813.22. Kadonosono K, Yazama F, Itoh N, et al. Expression of matrix metalloproteinase-7 in choroidal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1999. 128:382–384.23. Lambert V, Munaut C, Jost M, et al. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol. 2002. 161:1247–1253.24. Jin UH, Lee JY, Kang SK, et al. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005. 77:2760–2769.25. Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000. 45:115–134.26. Eichler W, Reiche A, Yafai Y, et al. Growth-related effects of oxidant-induced stress on cultured RPE and choroidal endothelial cells. Exp Eye Res. 2008. 87:342–348.27. Kuroki M, Voest EE, Amano S, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996. 98:1667–1675.28. Kannan R, Zhang N, Sreekumar PG, et al. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006. 12:1649–1659.29. Zang LY, Cosma G, Gardner H, et al. Effect of chlorogenic acid on hydroxyl radical. Mol Cell Biochem. 2003. 247:205–210.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Availability of Optical Coherence Tomography in Diagnosis and Classification of Choroidal Neovascularization
- The Spermatogenic Effect of Yacon Extract and Its Constituents and Their Inhibition Effect of Testosterone Metabolism
- Identification of proteins regulated by chlorogenic acid in an ischemic animal model: a proteomic approach
- A Case of Idiopathic Choroidal Neovascularization
- Laser-Induced Choroidal Neovascularization in Central Serous Chorioretinopathy: 4 Cases