Korean J Radiol.
2001 Mar;2(1):42-51. 10.3348/kjr.2001.2.1.42.
Radiologic-Pathologic Correlation of Unusual Lingual Masses:Part II: Benign and Malignant Tumors
- Affiliations
-
- 1Seoul Natl Univ Hosp,Dept Diagnost Radiol Chongno Gu,28 Yongon Dong, Seoul 110744, South Korea.
- KMID: 754122
- DOI: http://doi.org/10.3348/kjr.2001.2.1.42
Abstract
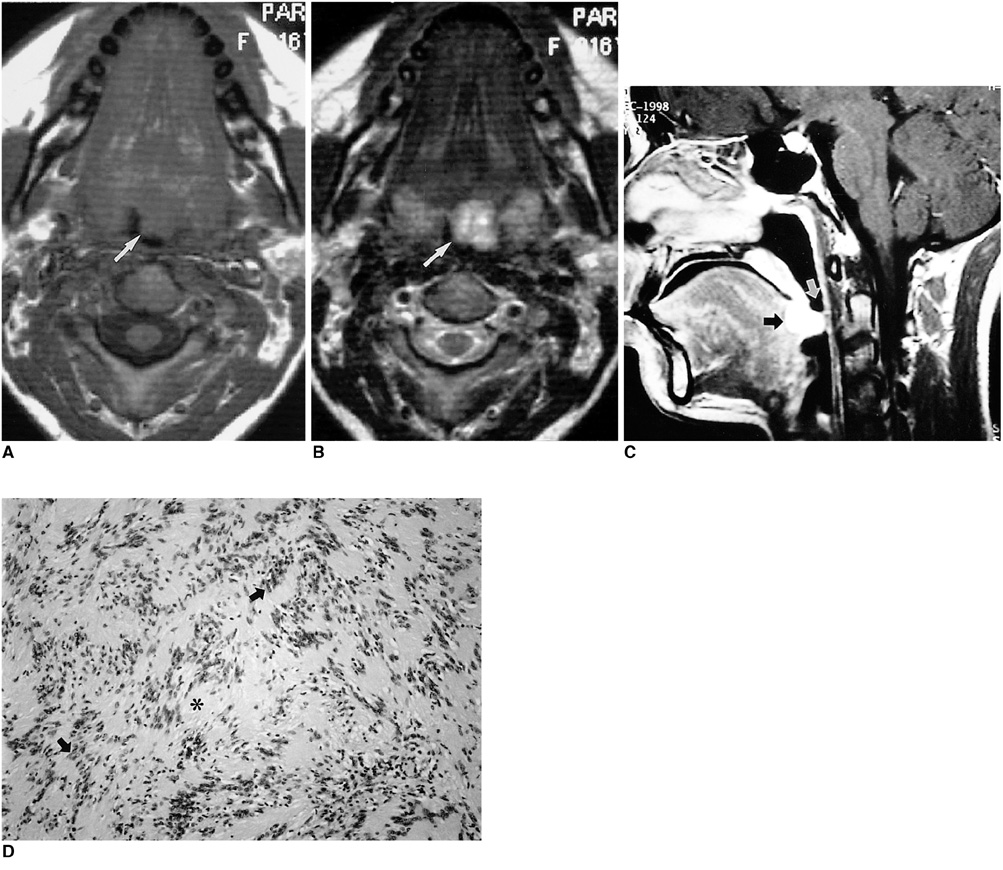
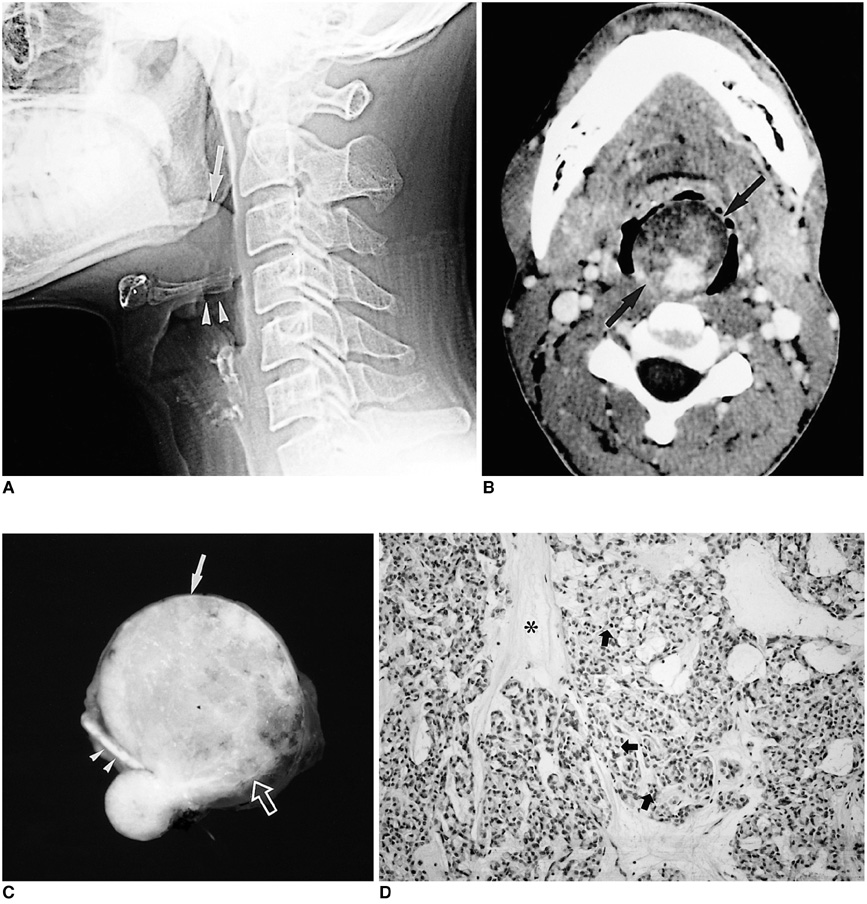
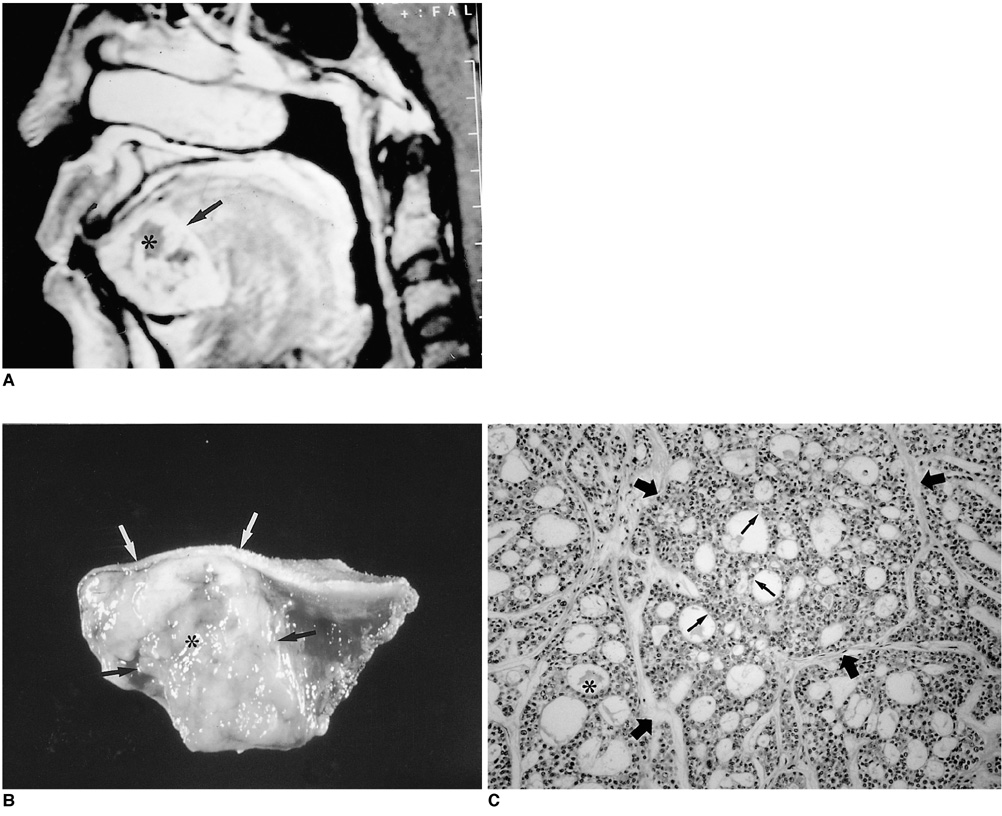
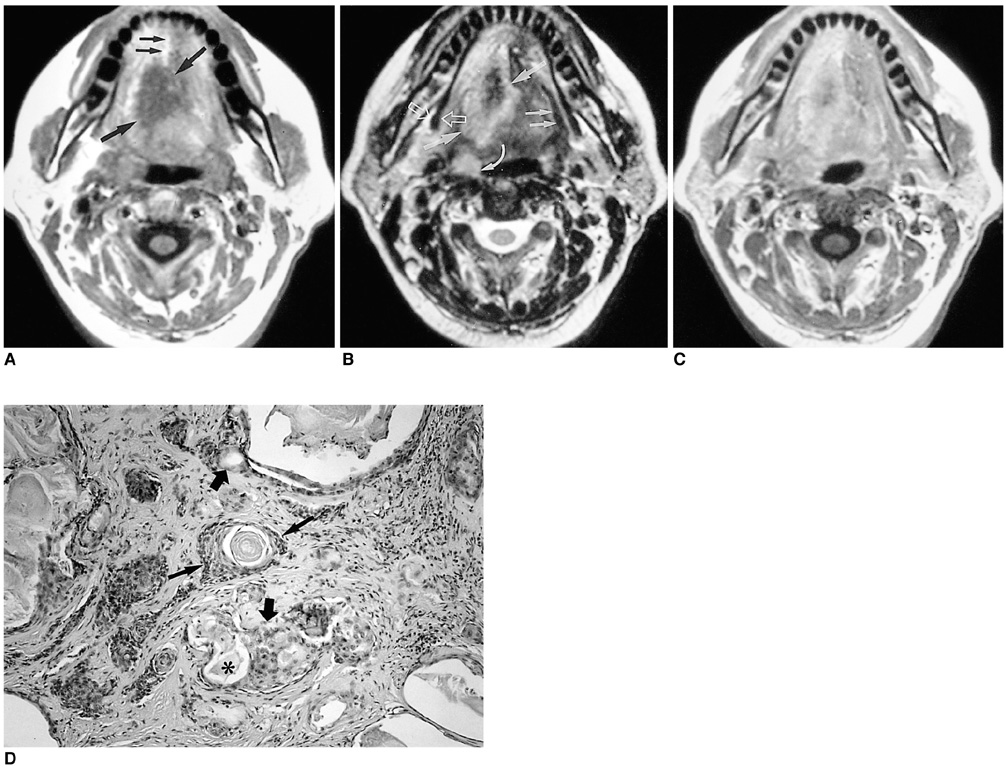
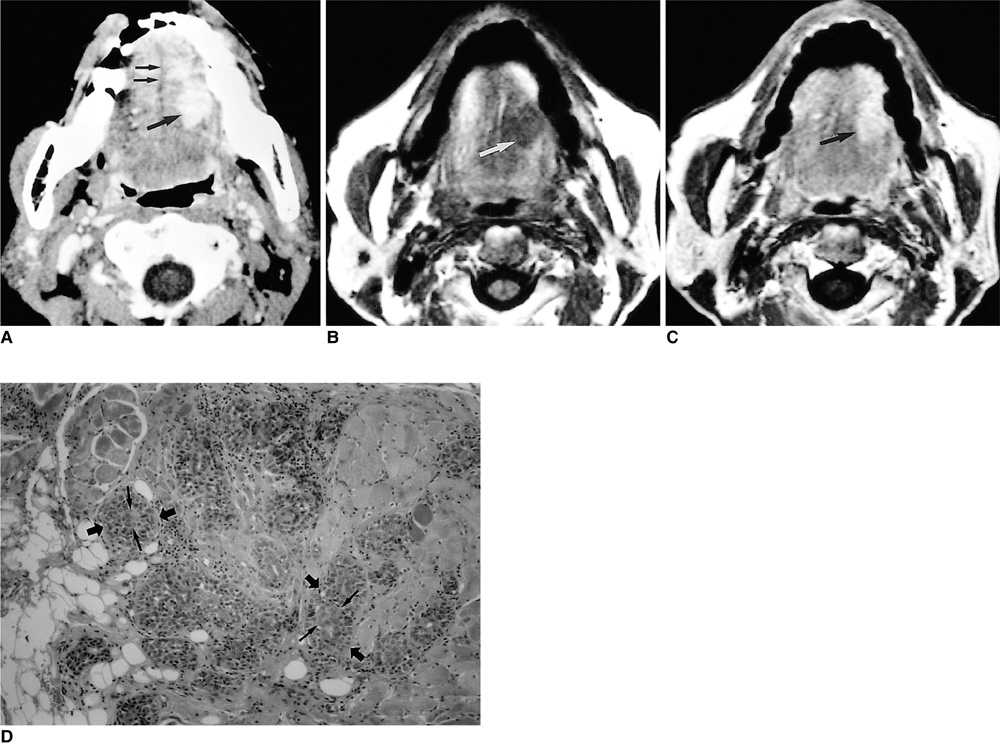
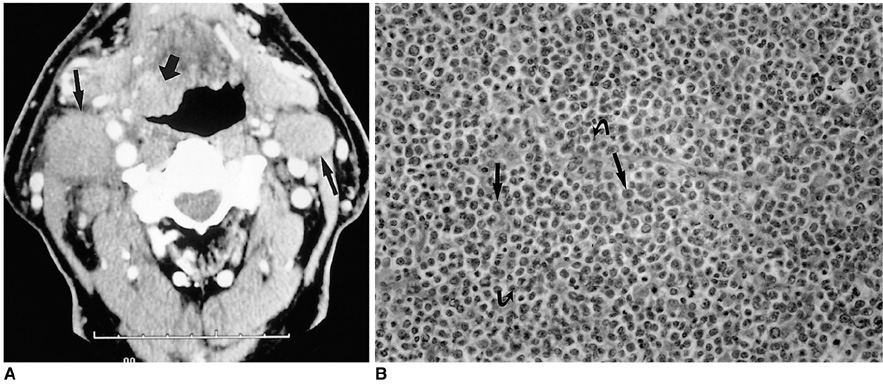
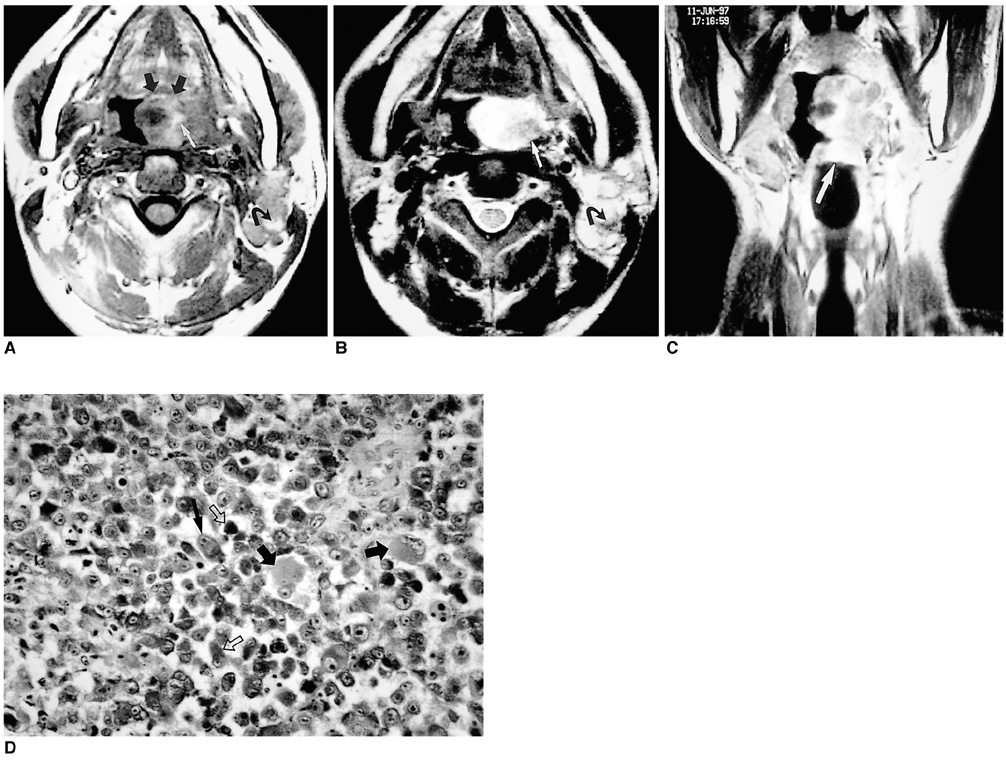
- Because the tongue is superficially located and the initial manifestation of most diseases occurring there is mucosal change, lingual lesionscan be easily accessed and diagnosed without imaging analysis. Some lingual neoplasms, however, may manifest as a submucosal bulge and be located in a deep portion of the tongue, such as its base; their true characteristics and extent may be rec-ognized only on cross-sectional images such as those obtained by CT or MRI. Some uncommon tongue neoplasms may have characteristic radiologic fea-tures, thus permitting quite specific radiologic diagnosis. Lipomas typically manifest at both CT and MR imaging as homogeneous nonenhancing lesions. Relative to subcutaneous fat they are isoattenuating on CT images, and all MR sequences show them as isointense. Due to the paramagnetic properties of melanin, metastases from melanotic melanoma usually demonstrate high signal intensity on T1-weighted MR images and low signal intensity on T2-weighted images. Although the radiologic findings for other submucosal neoplasms are nonspecific, CT and MR imaging can play an important role in the diagnostic work-up of these unusual tumors. Delineation of the extent of the tumor, and recognition and understanding of the spectrum of imaging and the pathologic features of these lesions, often help narrow the differential diagnosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Som PM, Scherl MP, Rao VM, Biller HF. Rare presentations of ordinary lipomas of the head and neck: a review. AJNR. 1986. 7:657–664.2. Dreher A, Gutmann R, Grevers G. Extracranial schwannoma of the ENT region. Review of the literature with a case report of benign schwannoma of the base of the tongue. HNO. 1997. 45:468–471. [Article in German].3. Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: report of 23 cases. Cancer. 1982. 49:562–572.4. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982. 69:412–420.5. Burrows PE, Mulliken JB, Fellows KE, Strand RD. Childhood hemangiomas and vascular malformations: angiographic differentiation. AJR. 1983. 141:483–488.6. Sigal R, Monnet O, De Baere T, et al. Adenoid cystic carcinoma of the head and neck: evaluation with MR imaging and clinical-pathologic correlation in 27 patients. Radiology. 1992. 184:95–101.7. Healey WV, Perzin KH, Smith L. Mucoepidermoid carcinoma of salivary gland origin: Classification, clinical-pathologic correlation, and results of treatment. Cancer. 1970. 26:368–388.8. Corio RL. Ellis G, Auclair P, Gnepp D, editors. Epithelial-myoepithelial carcinoma. Surgical pathology of the salivary glands. 1991. Philadelphia, Pa.: WB Saunders Co.;100–109.9. Griffin TJ, Hurst PS, Swanson J. Non-Hodgkin's lymphoma: a case involving four third molar extraction sites. Oral Surg Oral Med Oral Pathol. 1988. 65:671–674.10. Zegarelli DJ, Tsukada Y, Pickren JW, Greene GW Jr. Metastatic tumor to the tongue: Report of twelve cases. Oral Surg Oral Med Oral Pathol. 1973. 35:202–211.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Review of Epithelial Tumors in the Lacrimal Gland
- Radiologic-Pathologic Correlation of Unusual Lingual Masses:Part I: Congenital Lesions
- Incidental Solid Renal Masses: Radiologic Assessment and Managements
- Usefulness of the Bosniak Classification in Cystic Renal Mass on CT
- The Comparison between Pre- and Postoperative Diagnosis in Renal Masses Smaller than 3cm in Diameter