Korean J Radiol.
2001 Dec;2(4):222-230. 10.3348/kjr.2001.2.4.222.
MR Imaging of Central Diabetes Insipidus: A Pictorial Essay
- Affiliations
-
- 1Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine. hklee2@www.amc.seoul.kr
- 2Department Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine.
- 3Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine.
- 4Department of Radiology , Samsung Medical Center, Sungkyunkwan University College of Medicine.
- KMID: 754092
- DOI: http://doi.org/10.3348/kjr.2001.2.4.222
Abstract
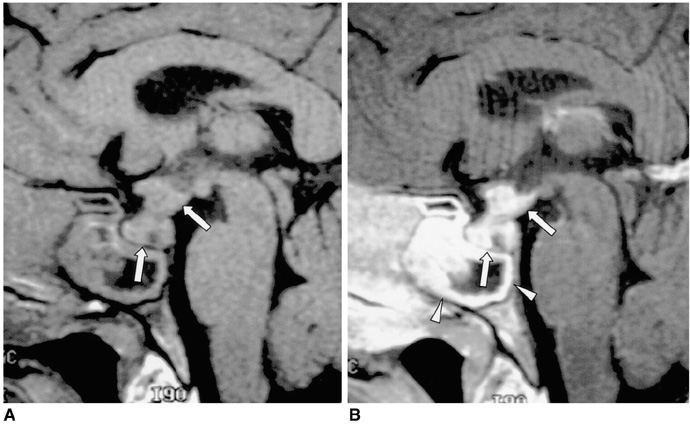
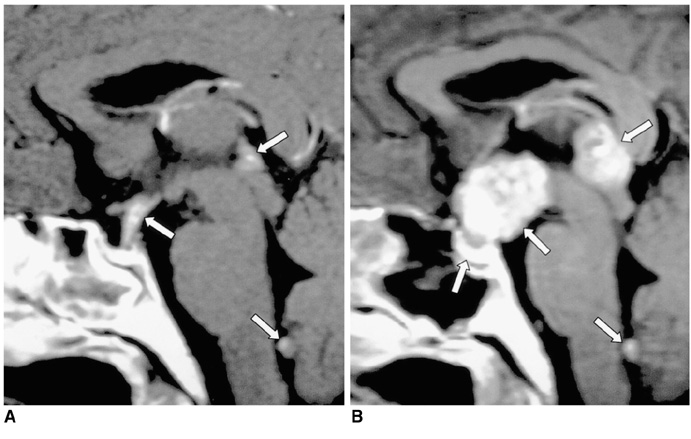
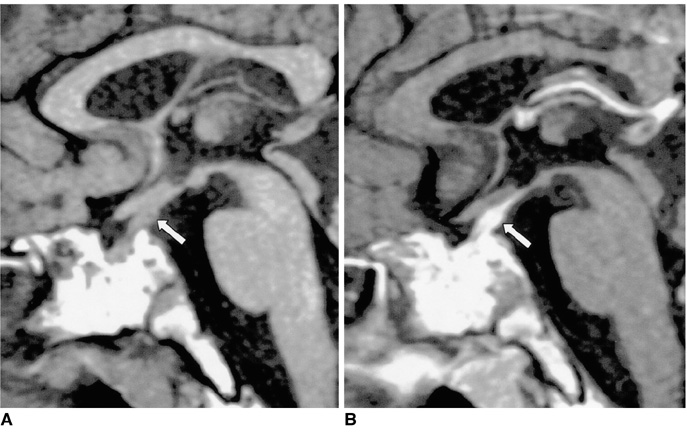
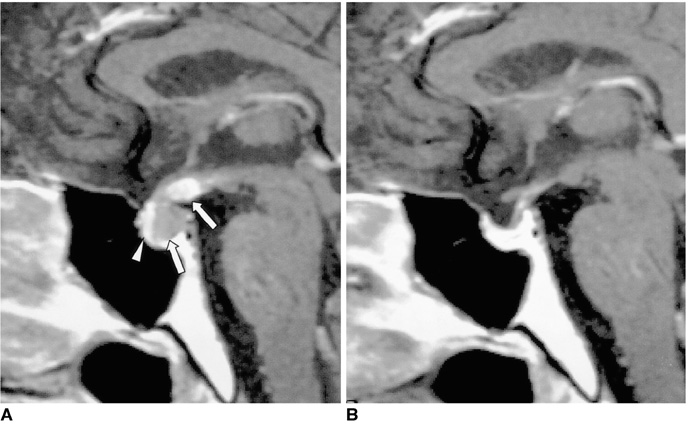
- Central diabetes insipidus (DI) can be the outcome of a number of diseases that affect the hypothalamic-neurohypophyseal axis. The causes of the condition can be classified as traumatic, inflammatory, or neoplastic. Traumatic causes include postoperative sella or transection of the pituitary stalk, while infectious or inflammatory causes include meningitis, lymphocytic hypophysitis, and granulomatous inflammations such as sarcoidosis and Wegener's granulomatosis. Various neoplastic conditions such as germinoma, Langerhans cell histiocytosis, metastasis, leukemic infiltration, lymphoma, teratoma, pituitary adenoma, craniopharyngioma, Rathke cleft cyst, hypothalamic glioma, and meningioma are also causes of central DI. In affected patients, careful analysis of these MR imaging features and correlation with the clinical manifestations can allow a more specific diagnosis, which is essential for treatment.
MeSH Terms
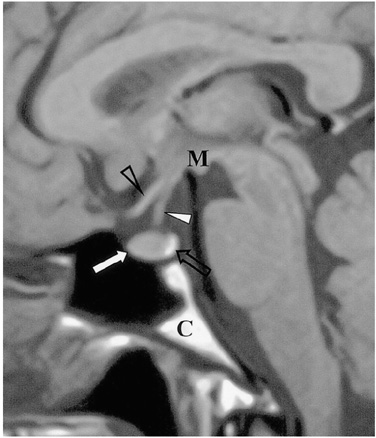
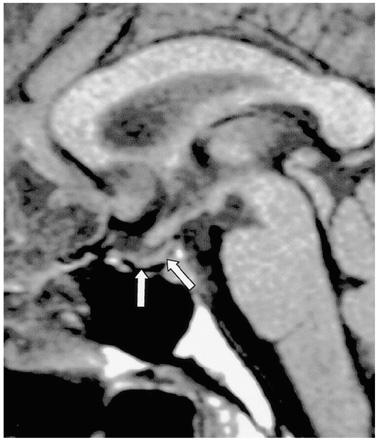
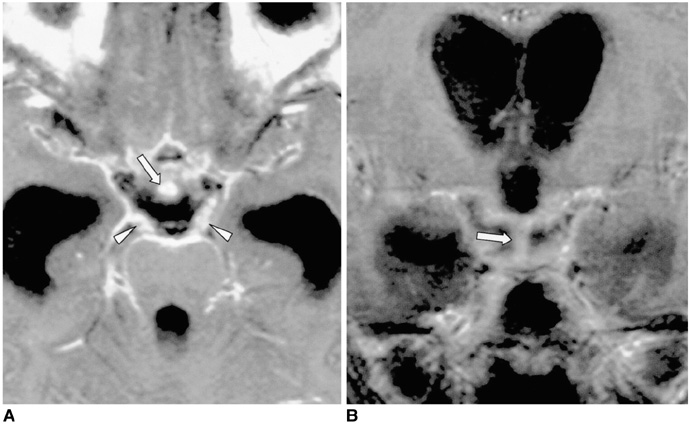
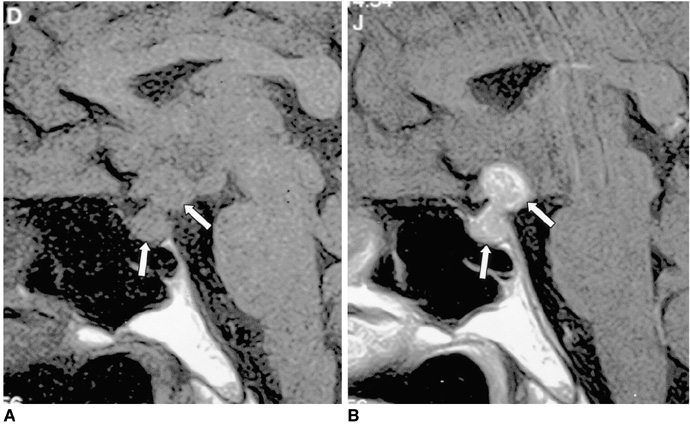
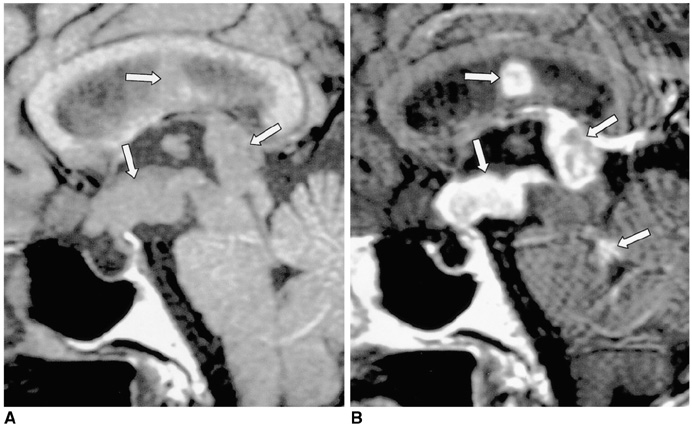
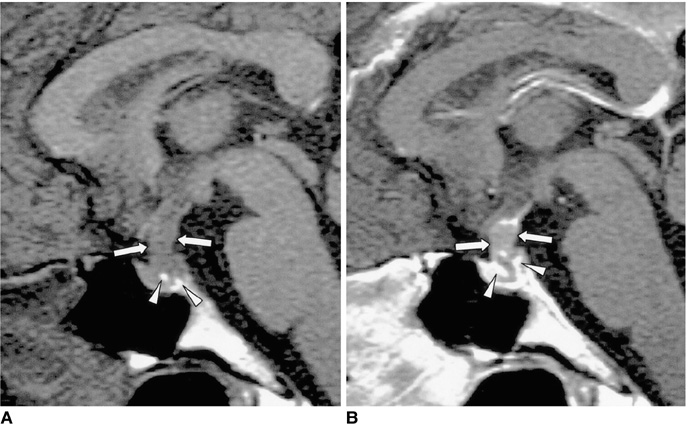
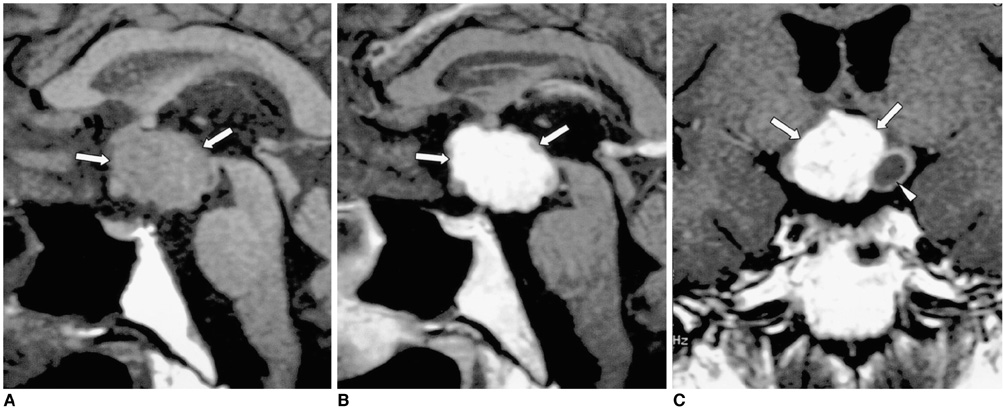
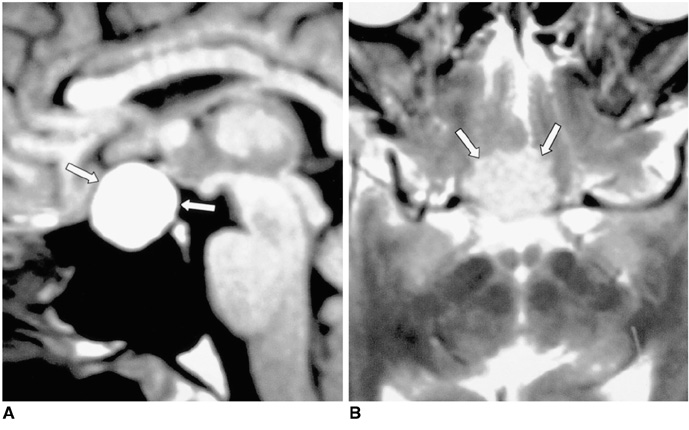
Figure
Cited by 1 articles
-
Pituitary Teratoma Presenting as Central Diabetes Insipidus with a Normal MRI Finding
Young Soo Kim, Seok Gu Kang, Young OK Kim
Yonsei Med J. 2010;51(2):293-294. doi: 10.3349/ymj.2010.51.2.293.
Reference
-
1. Ozata M, Tayfun C, Kurtaran K, et al. Magnetic resonance imaging of posterior pituitary for evaluation of the neurohypophyseal function in idiopathic and autosomal dominant neurohypophyseal diabetes insipidus. Eur Radiol. 1997. 7:1098–1102.2. Kucharczyk W, Lenkinski RE, Kucharczyk J, Henkelman RM. The effect of phospholipid vesicles on the NMR relaxation of water: an explanation for the MR appearance of the neurohypophysis? AJNR. 1990. 11:693–700.3. Ma L, Gao Y, Cai Y, Li T, Liang Y. MR evaluation of the brain in central diabetes insipidus. Chin Med J. 1996. 109:724–729.4. Tien R, Kucharczyk J, Kucharczyk W. MR imaging of the brain in patients with diabetes insipidus. AJNR. 1991. 12:533–542.5. Black PM, Zervas NT, Candia GL. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987. 20:920–924.6. Suh DC, Park JS, Park SK, Lee HK, Chang KH. Pituitary hemorrhage as a complication of hantaviral disease. AJNR. 1995. 16:175–178.7. Ahmed SR, Aiello DP, Page R, Hopper K, Towfighi J, Santen RJ. Necrotizing infundibulo-hypophysitis: a unique syndrome of diabetes insipidus and hypopituitarism. J Clin Endocrinol Metab. 1993. 76:1499–1504.8. Maghnie M. Lymphocytic hypophysitis and central diabetes insipidus during adolescence: what are the criteria for diagnosis? Eur J Pediatr. 1998. 157:693–694.9. Imura H, Nakao K, Shimatsu A, et al. Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med. 1993. 329:683–689.10. Maghnie M, Genovese E, Sommaruga MG, et al. Evolution of childhood central diabetes insipidus into panhypopituitarism with a large hypothalamic mass: is lymphocytic 'infundibuloneurohypophysitis' in children a different entity? Eur J Endocrinol. 1998. 139:635–640.11. Vomacka Z, Ehrmann J, Skvarilova M, Krc I. Granulomatous vasculitis of Churg-Strauss type in a patient with diabetes insipidus. Acta Univ Palacki Olomuc Fac Med. 1993. 135:17–19.12. Tien RD, Newton TH, McDermott MW, Dillon WP, Kucharczyk J. Thickened pituitary stalk on MR images in patients with diabetes insipidus and Langerhans cell histiocytosis. AJNR. 1990. 11:703–708.13. Szuwart U, Konig HJ, Bennefeld H, Weritz C, Kleinhans G. Clinical aspects of hypophyseal metastases. Onkologie. 1988. 11:66–69.14. Ra'anani P, Shpilberg O, Berezin M, Ben-Bassat I. Acute leukemia relapse presenting as central diabetes insipidus. Cancer. 1994. 73:2312–2316.15. Balmaceda CM, Fetell MR, Selman JE, Seplowitz AJ. Diabetes insipidus as first manifestation of primary central nervous system lymphoma. Neurology. 1994. 44:358–359.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Central Diabetes Insipidus Associated with Brachycephaly
- Morphea and Verruca Plana Complicated in Central Diabetes Insipidus
- A Case of Central Diabetes Insipidus Developed during Puerperium
- Imaging Features of the Mesenchymal Tumors of the Breast according to WHO Classification: A Pictorial Essay
- A Case of Hyperglycemic Hyperosmolar Syndrome in a Patient with Central Diabetes Insipidus and Type 2 Diabetes Mellitus