Korean J Radiol.
2001 Dec;2(4):210-215. 10.3348/kjr.2001.2.4.210.
Detection of Malignant Primary Hepatic Neoplasms with Gadobenate Dimeglumine (Gd-BOPTA) Enhanced T1-Weighted Hepatocyte Phase MR Imaging: Results of Off-site Blinded Review in a Phase-II Multicenter Trial
- Affiliations
-
- 1The Division of Abdominal Imaging and Intervention, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, USA. saini.sanjay@mgh.harvard.edu
- 2Department of Radiology, University of Pittsburgh, Pittsburgh, USA.
- 3Department of Radiology, Charite Charite Hospital, Humboldt University, Germany.
- 4Istituto di Radiologia, Policlinico di Borgo Roma, Verona, Italy.
- 5Dipartimento di Scienze Mediche, Oncologiche e Radiologiche, Universita degli Studi di Modena e Reggio Emilia, Modena, Italy.
- 6Istituto di Radiologia, Policlinico San Matteo, Pavia, Italy.
- 7Istituto di Scienze Radiologiche e Formazione dell'Imagine, Ospedale Santissima Annunziata, Chieti, Italy.
- 8Service de Radiologie, Hopital Henri Mondor, Creteil, France.
- 9Department of Diagnostic Radiology, University Hospital of Wales, United Kingdom.
- 10Medical and Regulatory Affairs, Bracco SpA, Via E. Folli 50, Milan, Italy.
- KMID: 754090
- DOI: http://doi.org/10.3348/kjr.2001.2.4.210
Abstract
OBJECTIVE
To investigate the efficacy of gadobenate dimeglumine (Gd-BOPTA) enhanced MR imaging for the detection of liver lesions in patients with primary malignant hepatic neoplasms. MATERIALS AND METHODS: Thirty-one patients with histologically proven primary malignancy of the liver were evaluated before and after administration of Gd-BOPTA at dose 0.05 or 0.10 mmol/kg. T1-weighted spin echo (T1W-SE) and gradient echo (T1W-GRE) images were evaluated for lesion number, location, size and confidence by three off-site independent reviewers and the findings were compared to reference standard imaging (intraoperative ultrasound, computed tomography during arterial portography or lipiodol computed tomography). Results were analyzed for significance using a two-sided McNemar's test. RESULTS: More lesions were identified on Gd-BOPTA enhanced images than on unenhanced images and there was no significant difference in lesion detection between either concentration. The largest benefit was in detection of lesions under 1 cm in size (7 to 21, 9 to 15, 16 to 18 for reviewers A, B, C respectively). In 68% of the patients with more than one lesion, Gd-BOPTA increased the number of lesions detected. CONCLUSION: Liver MR imaging after Gd-BOPTA increases the detection of liver lesions in patients with primary malignant hepatic neoplasm.
MeSH Terms
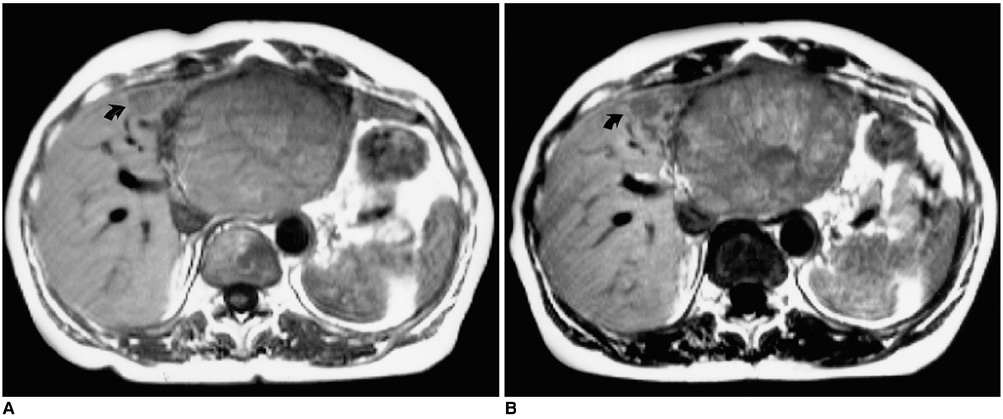
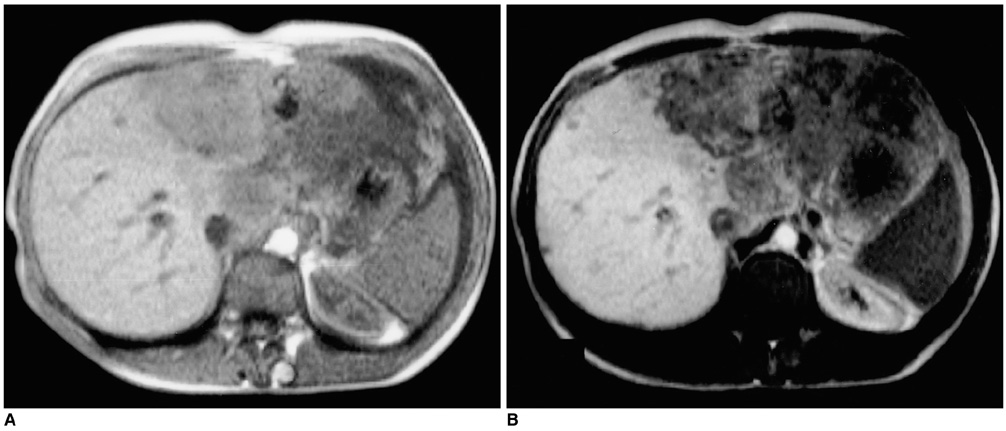
Figure
Reference
-
1. Miller WJ, Baron RL, Dodd GD III, Federle MP. Malignancies in patients with cirrhosis: CT sensitivity in 200 consecutive transplant patients. Radiology. 1994. 193:645–650.2. Dodd GD III, Miller WJ, Baron RL, Skolnick ML, Campbell WL. Detection of malignant tumors in end-stage cirrhotic livers: Efficacy of sonography as a screening technique. AJR. 1992. 159:727–733.3. Nelson RC, Chezmar JL, Sugarbaker PH, Bernadino ME. Hepatic tumors: Comparison of CT during arterial portography, delayed CT, and MR imaging for preoperative evaluation. Radiology. 1989. 171:47–51.4. Baron RL. Understanding and optimizing use of contrast material for CT of the liver. AJR. 1994. 163:323–331.5. Taourel PG, Pageaux GP, Coste V, et al. Small hepatocellular carcinoma in patients undergoing liver transplantation: detection with CT after injection of iodized oil. Radiology. 1995. 197:377–380.6. Manfredi R, Maresca G, Baron RL, et al. Gadobenate Dimeglumine [BOPTA] enhanced MR imaging: Patterns of enhancement in normal liver and cirrhosis. J Magn Reson Imaging. 1998. 8:862–867.7. Caudana R, Morana G, Pirovano GP, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium Benzyloxypropoinictetra- acetate (BOPTA)- Preliminary results of phase II clinical application. Radiology. 1996. 199:513–520.8. Vogl TJ, Stupavsky A, Pegios W, et al. Hepatocellular carcinoma: Evaluation with dynamic and static Gadobenate Dimeglumine-enhanced MR imaging and histopathologic correlation. Radiology. 1997. 205:721–728.9. Murakami T, Baron RL, Peterson MS, et al. Hepatocellular carcinoma: MR imaging with Mangafodipir Trisodium. Radiology. 1996. 200:69–77.10. Petersein J, Spinazzi A, Giovagnoni A, et al. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging- a multicenter phase III clinical study. Radiology. 2000. 215:727–736.11. Kirchin MA, Pirovano G, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA): an overview. Invest Radiol. 1999. 33:798–809.12. Vittadini G, Felder E, Musu C, Tirone C. Preclincial profile of Gd-BOPTA; a liver specific MRI contrast agent. Invest Radiol. 1990. 25:S59–S60.13. De Haen C, Gozzini L. Soluble-type hepatobiliary contrast agents for MR imaging. J Magn Reson Imaging. 1993. 3:179–183.14. Spinazzi A, Lorusso V, Pirovano G, Taroni P, Kirchin M, Davies A. MultiHance clinical pharmacology: biodistribution and MR enhancement of the liver. Acad Radiol. 1998. 5:S. 86–89.15. Spinazzi A, Lorusso V, Pirovano G, Kirchin M. Safety, tolerability, biodistribution and MR enhancement of the liver with Gd-BOPTA: results of clinical pharmacology and pilot imaging studies in non-patient and patient volunteers. Acad Radiol. 1999. 6:282–291.16. Vogl TJ, Pegios W, McMahon C, Balzer J, et al. Gadobenate Dimeglumine- a new contrast agent for MR imaging: Preliminary evaluation in healthy volunteers. AJR. 1992. 158:887–892.17. Rosati G, Pirovano G, Spinazzi A. Interim results of phase II clinical testing of Gadobenate Dimeglumine. Invest Radiol. 1994. 29:S. S183–S185.18. Slater GJ, Saini S, Mayo-Smith W, Sharma P, et al. Mn-MPDP enhanced MR imaging of the liver: analysis of pulse sequence performance. Clin Radiol. 1996. 51:484–486.19. Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology. 1996. 201:337–345.20. Hamm B, Mahfouz AE, Taupitz M, et al. Liver metastases: improved detection with dynamic gadolinium-enhanced MR imaging. Radiology. 1997. 202:677–682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic Enhancement on Gd-BOPTA-enhanced MR Imaging: Comparison between Cirrhotic and Normal Livers
- Gadobenate Dimeglumine-enhanced MR of VX2 Carcinoma in Rabbit Liver: Usefulness of the Delayed Phase Imaging and Optimal Pulse Sequence
- Hypointense Hepatic Lesions Depicted on Gadobenate Dimeglumine-Enhanced Three-Hour Delayed Hepatobiliary-Phase MR Imaging: Differentiation between Benignancy and Malignancy
- Enhancement Pattern of Focal Hepatic Tumors with Gadobenate Dimeglumine-Enhanced Delayed MR Imaging
- Gadobenate Dimeglumine as an Intrabiliary Contrast Agent: Comparison with Mangafodipir Trisodium with Respect to Non-dilated Biliary Tree Depiction