Korean J Radiol.
2009 Jun;10(3):294-302. 10.3348/kjr.2009.10.3.294.
Hypointense Hepatic Lesions Depicted on Gadobenate Dimeglumine-Enhanced Three-Hour Delayed Hepatobiliary-Phase MR Imaging: Differentiation between Benignancy and Malignancy
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. kshyun@skku.edu
- KMID: 1779457
- DOI: http://doi.org/10.3348/kjr.2009.10.3.294
Abstract
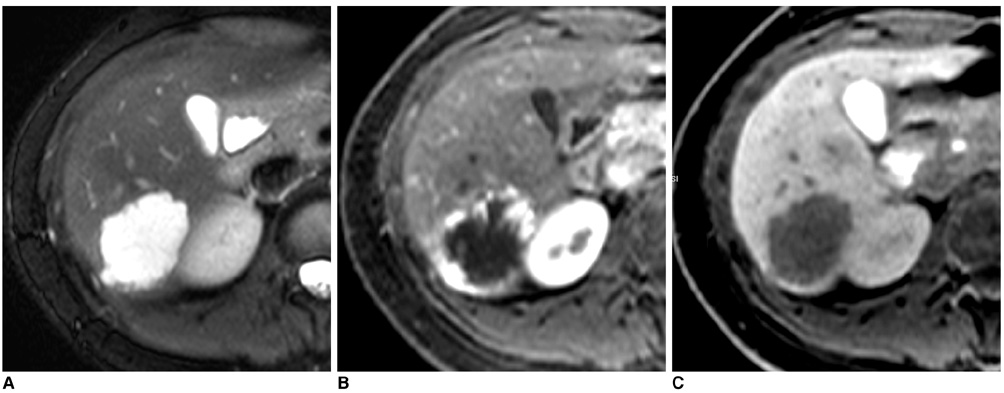
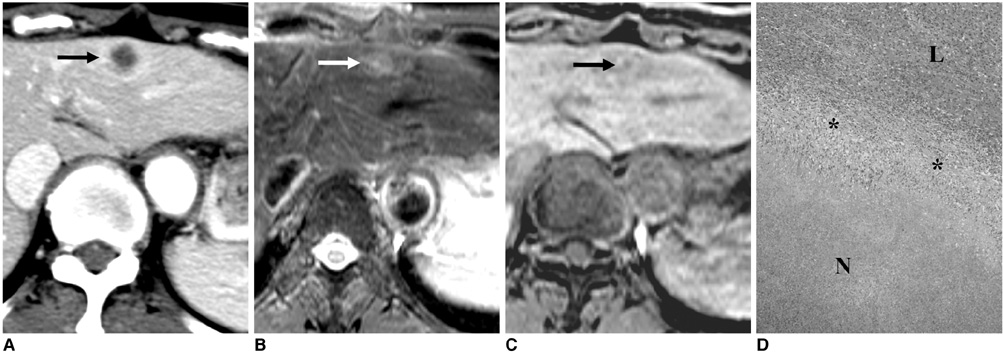
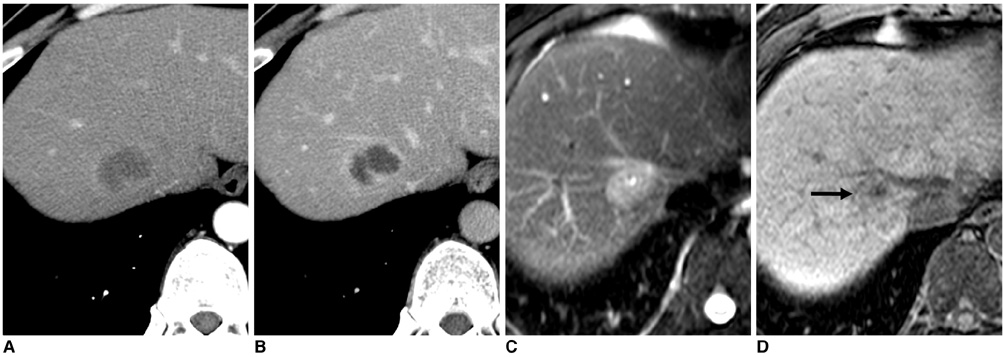
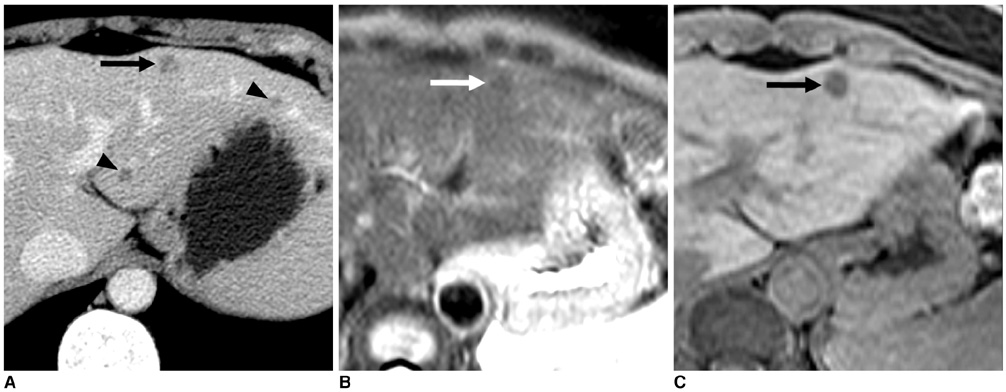
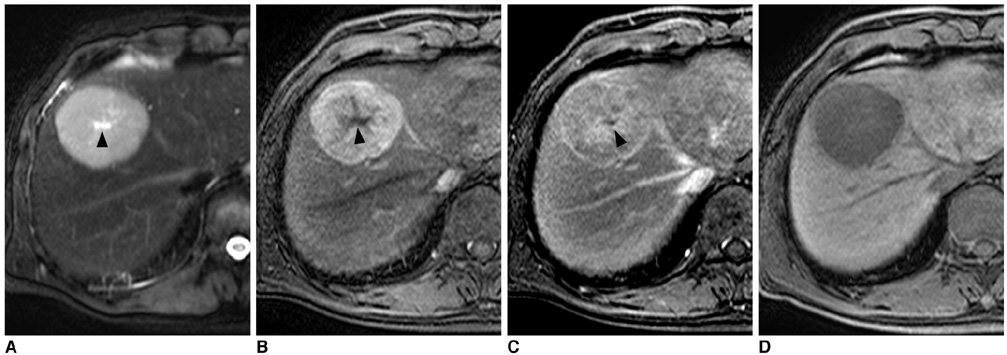
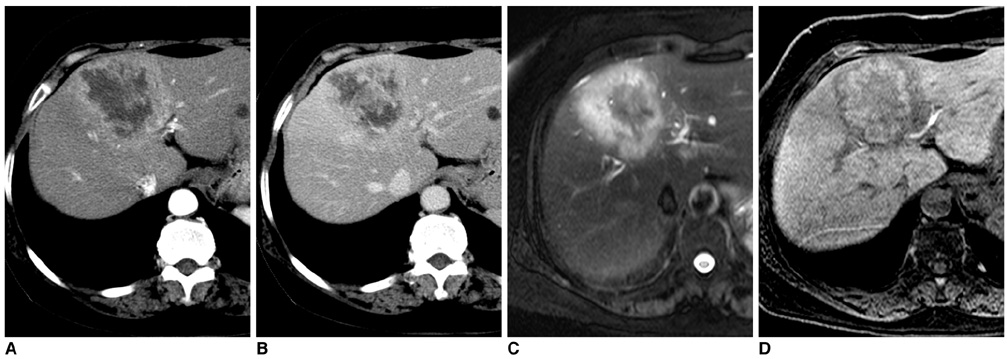
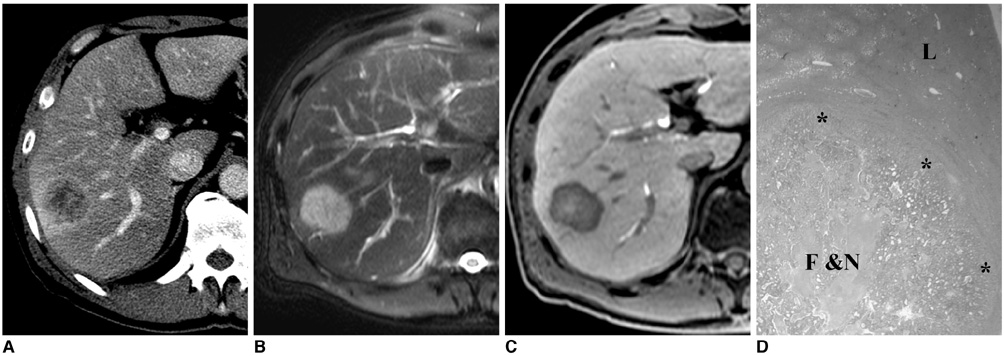
- Gadobenate dimeglumine-enhanced magnetic resonance (MR) imaging simultaneously provides both morphological and functional information by the acquisition of dynamic and hepatobiliary-phase imaging. Focal lesions with no functioning hepatocytes, where hepatobiliary metabolism is blocked or inhibited, are generally unable to uptake and excrete gadobenate dimeglumine into the bile. Such lesions are typically malignant and usually appear hypointense as compared to the normal liver parenchyma as seen on hepatobiliary-phase imaging. However, various benign hepatic lesions may also be hypointense due to (a) the presence of no functioning hepatocytes, (b) damage to the functioning hepatocytes or (c) impairment of biliary function as depicted on hepatobiliary-phase imaging. All of these imaging features may result in recognition of the benign hepatic lesions as hepatic malignancies. As depicted on three-hour delayed hepatobiliary-phase imaging, peripheral iso/hyperintensity due to fibrotic tissue compared to the hypointense center with a fuzzy margin may be a clue for the presence of a benign hepatic lesion. In contrast, peripheral hypointensity due to rich tumoral cellularity compared to the center with a clear margin may favor an indication of the presence of a malignant hepatic lesion.
MeSH Terms
-
Adult
Aged
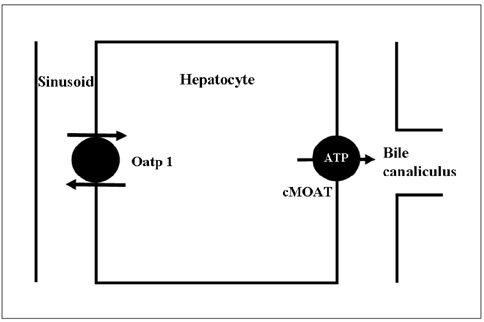
Contrast Media/*diagnostic use
Diagnosis, Differential
Female
Humans
Image Enhancement/methods
Liver/pathology
Liver Diseases/diagnosis/pathology
Liver Neoplasms/*diagnosis/*pathology
Magnetic Resonance Imaging/*methods
Male
Meglumine/*analogs & derivatives/diagnostic use
Middle Aged
Organometallic Compounds/*diagnostic use
Time
Figure
Reference
-
1. Cavagna FM, Maggioni F, Castelli PM, Daprà M, Imperatori LG, Lorusso V, et al. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol. 1997. 32:780–796.2. Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol. 1998. 33:798–809.3. Spinazzi A, Lorusso V, Pirovano G, Kirchin M. Safety, tolerance, biodistribution and MR imaging enhancement of the liver with gadobenate dimeglumine: results of clinical pharmacologic and pilot imaging studies in nonpatient and patient volunteers. Acad Radiol. 1999. 6:282–291.4. Bartolozzi C, Crocetti L, Lencioni R, Cioni D, Della Pina C, Campani D. Biliary and reticuloendothelial impairment in hepatocarcinogenesis: the diagnostic role of tissue-specific MR contrast media. Eur Radiol. 2007. 17:2519–2530.5. Petersein J, Spinazzi A, Giovagnoni A, Soyer P, Terrier F, Lencioni R, et al. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging-a multicenter phase III clinical study. Radiology. 2000. 215:727–736.6. Kim YK, Lee JM, Kim CS. Gadobenate dimeglumine-enhanced liver MR imaging: value of dynamic and delayed imaging for the characterization and detection of focal liver lesions. Eur Radiol. 2004. 14:5–13.7. Grazioli L, Morana G, Federle MP, Brancatelli G, Testoni M, Kirchin MA, et al. Focal nodular hyperplasia: morphologic and functional information from MR imaging with gadobenate dimeglumine. Radiology. 2001. 221:731–739.8. Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005. 236:166–177.9. Leen E. MultiHance-enhanced MRI in the characterisation of focal liver lesions. Eur Radiol. 2004. 14:O31–O35.10. Kim JI, Lee JM, Choi JY, Kim YK, Kim SH, Lee JY, et al. The value of gadobenate dimeglumine-enhanced delayed phase MR imaging for characterization of hepatocellular nodules in the cirrhotic liver. Invest Radiol. 2008. 43:202–210.11. Runge VM, Wells JW, Williams NM. Hepatic abscess. Magnetic resonance imaging findings using gadolinium-BOPTA. Invest Radiol. 1996. 31:781–788.12. Marin D, Iannaccone R, Catalano C, Passariello R. Multinodular focal fatty infiltration of the liver: atypical imaging findings on delayed T1-weighted Gd-BOPTA-enhanced liver-specific MR images. J Magn Reson Imaging. 2006. 24:690–694.13. Schneider G, Fries P, Samaras P, Remberger K, Uder M, Kramann B. Inflammatory pseudotumor of the liver in a patient with congenital granulocytopenia and HCV infection. Eur J Radiol. 2003. 48:293–298.14. Pascolo L, Petrovic S, Cupelli F, Bruschi CV, Anelli PL, Lorusso V, et al. Abc protein transport of MRI contrast agents in canalicular rat liver plasma vesicles and yeast vacuoles. Biochem Biophys Res Commun. 2001. 282:60–66.15. Pastor CM, Planchamp C, Pochon S, Lorusso V, Montet X, Mayer J, et al. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation. Radiology. 2003. 229:119–125.16. Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997. 26:1667–1677.17. Stanca C, Jung D, Meier PJ, Kullak-Ublick GA. Hepatocellular transport proteins and their role in liver disease. World J Gastroenterol. 2001. 7:157–169.18. Spinazzi A, Lorusso V, Pirovano G, Taroni P, Kirchin MA, Davies A. Multihance clinical pharmacology: biodistribution and MR enhancement of the liver. Acad Radiol. 1998. 5:S86–S89.19. Gabata T, Matsui O, Kadoya M, Yoshikawa J, Ueda K, Kawamori Y, et al. Delayed MR imaging of the liver: correlation of delayed enhancement of hepatic tumors and pathologic appearance. Abdom Imaging. 1998. 23:309–313.20. Mahfouz AE, Hamm B, Wolf KJ. Peripheral washout: a sign of malignancy on dynamic gadolinium-enhanced MR images of focal liver lesions. Radiology. 1994. 190:49–52.21. Nath DS, Rutzick AD, Sielaff TD. Solitary fibrous tumor of the liver. AJR Am J Roentgenol. 2006. 187:W187–W190.22. Fuksbrumer MS, Klimstra D, Panicek DM. Solitary fibrous tumor of the liver: imaging findings. AJR Am J Roentgenol. 2000. 175:1683–1687.23. Machida T, Takahashi T, Itoh T, Hirayama M, Morita T, Horita S. Reactive lymphoid hyperplasia of the liver: a case report and review of literature. World J Gastroenterol. 2007. 13:5403–5407.24. Takahashi H, Sawai H, Matsuo Y, Funahashi H, Satoh M, Okada Y, et al. Reactive lymphoid hyperplasia of the liver in a patient with colon cancer: report of two cases. BMC Gastroenterol. 2006. 6:25.25. Fauci AS, Harley JB, Roberts WC, Ferrans VJ, Gralnick HR, Bjornson BH. NIH conference. The idiopathic hypereosinophilic syndrome. Clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982. 97:78–92.26. Lee WJ, Lim HK, Lim JH, Kim SH, Choi SH, Lee SJ. Foci of eosinophil-related necrosis in the liver: imaging findings and correlation with eosinophilia. AJR Am J Roentgenol. 1999. 172:1255–1261.27. Lim JH, Lee KS. Eosinophilic infiltration in Korea: idiopathic? Korean J Radiol. 2006. 7:4–6.28. Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988. 208:558–564.29. Caudana R, Morana G, Pirovano GP, Nicoli N, Portuese A, Spinazzi A, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium benzyloxypropionictetra-acetate (BOPTA)-preliminary results of phase II clinical application. Radiology. 1996. 199:513–520.30. Muramatsu Y, Takayasu K, Moriyama N, Shima Y, Goto H, Ushio K, et al. Peripheral low-density area of hepatic tumors: CT-pathologic correlation. Radiology. 1986. 160:49–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Gadobenate Dimeglumine - Enhanced Hepatobiliary Phase MR Imaging on Predicting Histological Grade of Hepatocellular Carcinoma
- Gadobenate Dimeglumine-enhanced MR of VX2 Carcinoma in Rabbit Liver: Usefulness of the Delayed Phase Imaging and Optimal Pulse Sequence
- Enhancement Pattern of Focal Hepatic Tumors with Gadobenate Dimeglumine-Enhanced Delayed MR Imaging
- The MR imaging diagnosis of liver diseases using gadoxetic acid: Emphasis on hepatobiliary phase
- Hepatic Lymphoma Representing Iso-Signal Intensity on Hepatobiliary Phase, in Gd-EOB-DTPA-Enhanced MRI: Case Report