Korean J Radiol.
2001 Dec;2(4):192-196. 10.3348/kjr.2001.2.4.192.
Diffusion-Weighted MR Imaging in Biopsy-Proven Creutzfeldt-Jakob Disease
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, and Institute of Radiation Medicine, SNUMRC, Seoul, Korea. changkh@radcom.snu.ac.kr
- 2Department of Neurology, Seoul National University College of Medicine.
- KMID: 754087
- DOI: http://doi.org/10.3348/kjr.2001.2.4.192
Abstract
OBJECTIVE
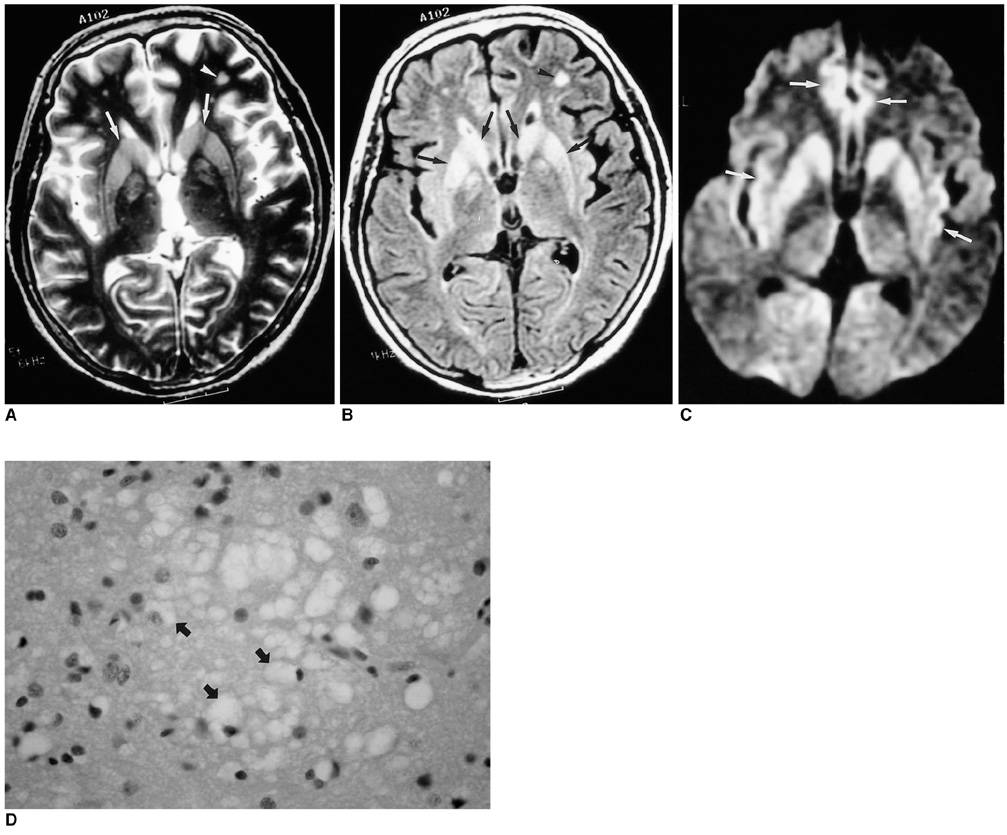
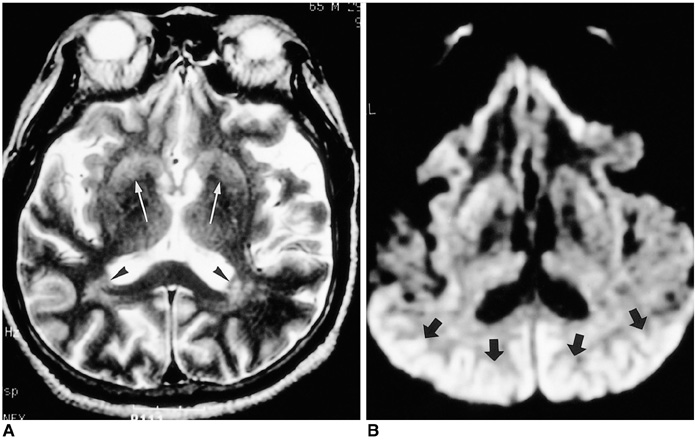
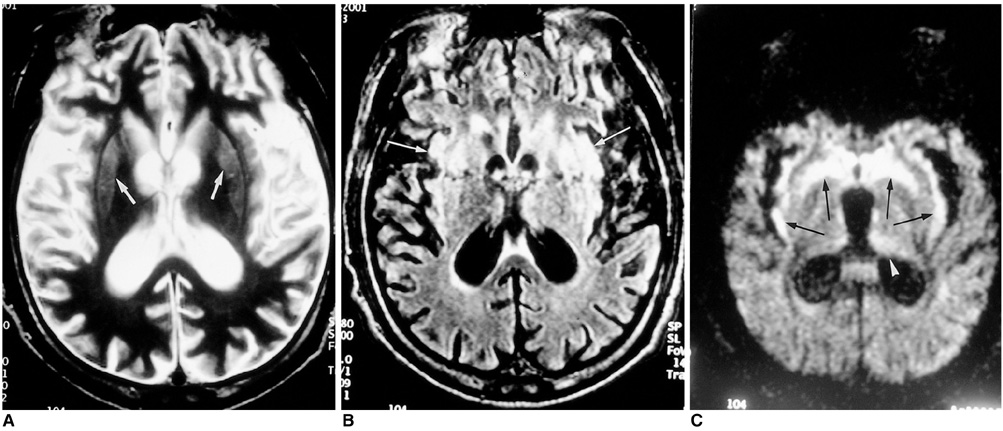
To compare conventional and diffusion-weighted MR imaging in terms of their depiction of the abnormalities occurring in Creutzfeldt-Jakob disease. MATERIALS AND METHODS: We retrospectively analyzed the findings of conventional (T2-weighted and fluid-attenuated inversion recovery) and diffusion-weighted MR imaging in four patients with biopsy-proven Creutzfeldt-Jakob disease. The signal intensity of the lesion was classified by visual assessment as markedly high, slightly high, or isointense, relative to normal brain parenchyma. RESULTS: Both conventional and diffusion-weighted MR images demonstrated bilateral high signal intensity in the basal ganglia in all four patients. Cortical lesions were observed on diffusion-weighted MR images in all four, and on fluid-attenuated inversion recovery MR images in one, but in no patient on T2-weighted images. Conventional MR images showed slightly high signal intensity in all lesions, while diffusion-weighted images showed markedly high signal intensity in most. CONCLUSION: Diffusion-weighted MR imaging is more sensitive than its conventional counterpart in the depiction of Creutzfeldt-Jakob disease, and permits better detection of the lesion in both the cerebral cortices and basal ganglia.
Keyword
MeSH Terms
Figure
Reference
-
1. Brown P, Cathala F, Castaigne P, Gajdusek DC. Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol. 1986. 20:597–602.2. Prusiner SB. Molecular biology of prion diseases. Science. 1991. 252:1515–1522.3. Finkenstaedt M, Szudra A, Zerr I, et al. MR imaging of Creutzfeldt-Jakob disease. Radiology. 1996. 199:793–798.4. Bahn MM, Kido DK, Lin W, Pearlman AL. Brain magnetic resonance diffusion abnormalities in Creutzfeldt-Jakob disease. Arch Neurol. 1997. 54:1411–1415.5. Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol. 1999. 56:577–583.6. Na DL, Suh CK, Choi SH, et al. Diffusion-weighted magnetic resonance imaging in probable Creutzfeldt-Jakob disease. Arch Neurol. 1999. 56:951–957.7. Demaerel P, Heiner L, Robberecht W, Sciot R, Wilms G. Diffusion-weighted MRI in sporadic Creutzfeldt-Jakob disease. Neurology. 1999. 52:205–208.8. Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Physiol. 1965. 42:288–292.9. Gideon P, Sorensen PS, Thomsen C, et al. Increased brain water self-diffusion in patients with idiopathic intracranial hypertension. AJNR. 1995. 16:381–387.10. Masters CL, Richardson EP Jr. Subacute spongiform encephalopathy (Creutzfeldt-Jakob disease): the nature and progression of spongiform change. Brain. 1978. 101:333–344.11. Zeidler M, Sellar RJ, Collie DA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet. 2000. 355:1412–1418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four Cases of Probable Creutzfeldt-Jacob Disease with High Signals in Cerebral Cortex on Diffusion Weighted MR Imaging
- Diffusion-Weighted MRI in Creutzfeldt-Jakob Disease: Focus on the Cerebral Cortex and Chronologic Change
- Rapidly Aggravated Creutzfeldt-Jacob Disease: Autopsy-Proven Case
- A case of Creutzfeldt-Jakob disease
- White Matter Lesions in a Patient With Creutzfeldt-Jakob Disease