Korean J Radiol.
2002 Jun;3(2):87-97. 10.3348/kjr.2002.3.2.87.
Optimal Pulse Sequence for Ferumoxides-Enhanced MR Imaging Used in the Detection of Hepatocellular Carcinoma: A Comparative Study Using Seven Pulse Sequences
- Affiliations
-
- 1Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Korea. shkim@smc.samsung.co.kr
- 2Department of Radiology, Ulsan Boram Hospital, Korea.
- KMID: 754067
- DOI: http://doi.org/10.3348/kjr.2002.3.2.87
Abstract
OBJECTIVE
To identify the optimal pulse sequence for ferumoxides-enhanced magnetic resonance (MR) imaging in the detection of hepatocelluar carcinomas (HCCs).
MATERIALS AND METHODS
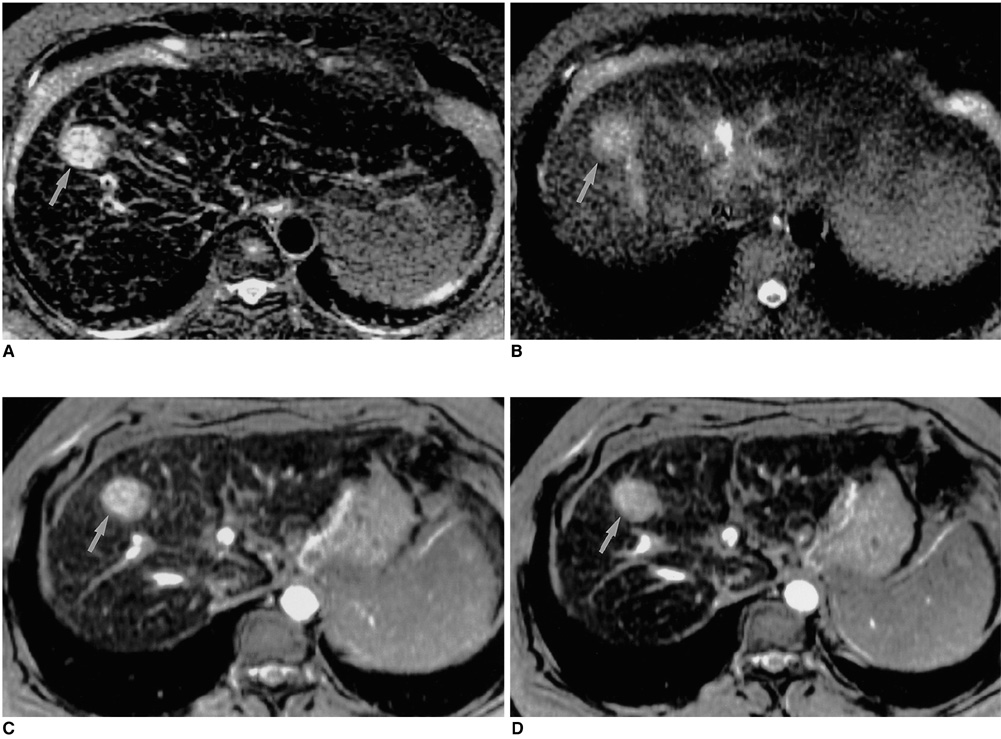
Sixteen patients with 25 HCCs underwent MR imaging following intravenous infusion of ferumoxides. All MR studies were performed on a 1.5-T MR system, using a phased-array coil. Ferumoxides (Feridex IV) at a dose of 15 micro mol/Kg was slowly infused intravenously, and axial images of seven sequences were obtained 30 minutes after the end of infusion. The MR protocol included fast spin-echo (FSE) with two echo times (TR3333-8571/TE18 and 90-117), singleshot FSE (SSFSE) with two echo times (TR infinity/TE39 and 98), T2*-weighted gradient-recalled acquisition in the steady state (GRASS) (TR216/TE20), T2*-weighted fast multiplanar GRASS (FMPGR) (TR130/TE8.4-9.5), and T2*-weighted fast multiplanar spoiled GRASS (FMPSPGR) (TR130/TE8.4-9.5). Contrast-to-noise ratios (CNRs) of HCCs determined during the imaging sequences formed the basis of quantitative analysis, and images were qualitatively assessed in terms of lesion conspicuity and image artifacts. The diagnostic accuracy of all sequences was assessed using receiver operating characteristic (ROC) analysis.
RESULTS
Quantitative analysis revealed that the CNRs of T2*-weighted FMPGR and T2*-weighted FMPSPGR were significantly higher than those of the other sequences, while qualitative analysis showed that image artifacts were prominent at T2*-weighted GRASS imaging. Lesion conspicuity was statistically significantly less clear at SSFSE imaging. In term of lesion detection, T2*-weighted FMPGR, T2*- weighted FMPSPGR, and proton density FSE imaging were statistically superior to the others.
CONCLUSION
T2*-weighted FMPGR, T2*- weighted FMPSPGR, and proton density FSE appear to be the optimal pulse sequences for ferumoxidesenhanced MR imaging in the detection of HCCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Saini S, Stark DD, Hahn PF, Wittenberg J, Brady TJ, Ferrucci JT. Ferrite particles: a superparamagnetic MR contrast agent for the reticuloendothelial system. Radiology. 1987. 162:211–216.2. Stark DD, Weissleder R, Elizondo G, et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology. 1988. 168:297–301.3. Kawamori Y, Matsui O, Kadoya M, Yoshikawa J, Demachi H, Takashima T. Differentiation of hepatocellular carcinoma from hyperplastic nodules induced in rat liver with ferrite-enhanced MR imaging. Radiology. 1992. 183:65–72.4. Vogl TJ, Hammerstingl R, Schwarz W, et al. Superparamagnetic iron oxide-enhanced versus gadolinium-enhanced MR imaging for differential diagnosis of focal liver lesions. Radiology. 1996. 198:881–887.5. Paley MR, Mergo PJ, Torres GM, Ros PR. Characterization of focal hepatic lesions with ferumoxides-enhanced T2-weighted MR imaging. AJR. 2000. 175:159–163.6. Lim JH, Choi D, Cho SK, et al. Conspicuity of hepatocellular nodular lesions in cirrhotic livers at ferumoxides-enhanced MR imaging: importance of Kupffer cell number. Radiology. 2001. 220:669–676.7. Nelson RC, Chezmar JL, Sugarbaker PH, Bernardino ME. Hepatic tumors: comparison of CT during arterial portography, delayed CT, and MR imaging for preoperative evaluation. Radiology. 1989. 172:27–34.8. Heiken JP, Weyman PJ, Lee LKT, et al. Detection of focal hepatic masses: prospective evaluation with CT, delayed CT, CT during arterial portography, and MR imaging. Radiology. 1989. 171:47–51.9. Soyer P, Levesque M, Elias D, Zeitoun G, Roche A. Detection of liver metastases from colorectal cancer: comparison of intraoperative ultrasound and CTAP. Radiology. 1992. 183:541–544.10. Soyer P, Levesque M, Caudron C, Elias D, Zeitoun G, Roche A. MRI of liver metastases from colorectal cancer vs CT during arterial portography. J Comput Assist Tomogr. 1993. 17:67–74.11. Choi D, Kim SH, Lim JH, et al. Preoperative detection of hepatocellular carcinoma: ferumoxides-enhanced MR imaging versus combined helical CT during arterial portography and CT hepatic arteriography. AJR. 2001. 176:475–482.12. Seneterre E, Taourel P, Bouveir Y, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996. 200:785–792.13. Oudkerk M, Heuvel AG, Wielopolski PA, Schitz PI, Rinkes IHMB, Wiggers T. Hepatic lesions: detection with ferumoxide-enhanced T1-weighted MR imaging. Radiology. 1997. 203:449–456.14. Yamamoto H, Tasuyuku Y, Yoshimatsu S, et al. Hepatocellular carcinoma in cirrhotic livers: detection with unenhanced and iron oxide-enhanced MR imaging. Radiology. 1995. 195:106–112.15. Hagspiel KD, Neidl KFW, Eichenberger AC, Weder W, Marinecek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995. 196:471–478.16. Ward J, Naik KS, Guthrie JA, Wilson D, Robinson PJ. Hepatic lesion detection: comparison of MR imaging after the administration of superparamagnetic iron oxide with dual-phase CT by using alternative-free response receiver operating characteristic analysis. Radiology. 1999. 210:459–466.17. Blakeborough A, Ward J, Wilson D, et al. Hepatic lesion detection at MR imaging: a comparative study with four sequences. Radiology. 1997. 203:759–765.18. Tanimoto A, Satoh Y, Yuasa Y, Jinzaki M, Hiramatsu K. Performance of Gd-EOB-DTPA and superparamagnetic iron oxide particles in the detection of primary liver cancer: a comparative study by alternative free-response receiver operating characteristic analysis. J Magn Reson Imaging. 1997. 7:120–124.19. Ros PR, Freeny PC, Harms SE, et al. Hepatic MR imaging with ferumoxides: a multicenter clinical trial of the safety and efficacy in the detection of focal hepatic lesions. Radiology. 1995. 196:481–488.20. Winter TC, Freeny PC, Nghiem HV, et al. MR imaging with IV superparamagnetic iron oxide: efficacy in the detection of focal hepatic lesions. AJR. 1993. 161:1191–1198.21. Fretz CJ, Elizondo G, Weissleder R, Hahn PF, Stark DD, Ferrucci JT. Superparamagnetic iron oxide-enhanced MR imaging: pulse sequence of optimization for detection of liver cancer. Radiology. 1989. 172:393–397.22. Bellin MF, Zaim S, Auberton E, et al. Liver metastases: safety and efficacy of detection with superparamagnetic iron oxide in MR imaging. Radiology. 1994. 193:657–663.23. Van Beers BE, Lacrosse M, Jacques J, et al. Detection and segmental location of malignant hepatic tumors: comparison of ferumoxides-enhanced gradient-echo and T2-weighted spin-echo MR imaging. AJR. 1997. 168:713–717.24. Yamamoto H, Yamashita Y, Yoshimatsu S, et al. Hepatocellular carcinoma in cirrhotic liver: detection with unenhanced and iron oxide-enhanced MR imaging. Radiology. 1995. 195:106–112.25. Arbab AS, Ichikawa T, Araki T, et al. Detection of hepatocellular carcinoma and its metastases with various pulse sequences using superparamagnetic iron oxide (SHU-555-A). Abdom Imaging. 2000. 25:151–158.26. Kanematsu M, Itoh K, Matsuo M, et al. Malignant hepatic tumor detection with ferumoxides-enhanced MR imaging with a 1.5-T system: comparison of four imaging pulse sequences. J Magn Reson Imaging. 2001. 13:249–257.27. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.28. Metz CE. Some practical issues of the experimental design and data analysis in radiological ROC studies. Invest Radiol. 1989. 24:234–245.29. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1992. 143:29–36.30. Tang Y, Yamashita Y, Arakawa A, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium-and ferumoxides-enhanced MR imaging. AJR. 1999. 172:1547–1554.31. Ward J, Chen F, Guthrie JA, et al. Hepatic lesion detection after superparamagnetic iron oxide enhancement: comparison of five T2-weighted sequences at 1.0 T by using alternative-free response receiver operating characteristic analysis. Radiology. 2000. 214:159–166.32. Schwartz LH, Seltzer SE, Tempany CM, et al. Superparamagnetic iron oxide hepatic MR imaging: efficacy and safety using conventional and fast spin-echo pulse sequences. J Magn Reson Imaging. 1995. 5:566–570.33. Abe Y, Yamashita Y, Namimoto T, Tang Y, Takahashi M. The value of fast and ultrafast T2-weighted MR imaging sequences in hepatic enhancement with ferumoxides: comparison with conventional spin-echo sequence. Radiat Med. 2000. 18:97–105.34. Semelka RC, Kelekis NL, Thomasson D, Brown MA, Laub GA. HASTE MR imaging: description of technique and preliminary results in the abdomen. J Magn Reson Imaging. 1996. 6:698–699.35. Grangier C, Tourniaire J, Mentha G, et al. Enhancement of liver hemangiomas on T1-weighted MR SE images by superparamagnetic iron oxide particles. J Comput Assist Tomogr. 1994. 18:888–896.36. Soyer P. Will ferumoxides-enhanced MR imaging replace CT during arterial portography in the detection of hepatic metastases? Prologue to a promising future. Radiology. 1996. 201:610–611.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mn-DPDP-enhanced MR Imaging: the Optimal Pulse Sequence for Detection of Focal Hepatic Tumor
- Double Contrast Media enhanced MRI with Ferumoxides-Gadolinium on Hepatocellular Carcinoma
- SPIO-enhanced MR Imaging for HCC Detection in Cirrhotic Patient: Comparison of Various Techniques for Optimal Sequence Selection
- In Vitro Imaging of MRI and Ultrasound for Gastric Carcinoma
- Pulse Sequence Optimization for Superparamagnetic Iron Oxide-enhanced MR Imaging in the Detection of Hepatic VX2 Tumors in Rabbits