Korean J Radiol.
2002 Sep;3(3):171-179. 10.3348/kjr.2002.3.3.171.
Perfusion MR Imaging: Clinical Utility for the Differential Diagnosis of Various Brain Tumors
- Affiliations
-
- 1Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hsbyun@smc.samsung.co.kr
- 2Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 754059
- DOI: http://doi.org/10.3348/kjr.2002.3.3.171
Abstract
OBJECTIVE
To determine the utility of perfusion MR imaging in the differential diagnosis of brain tumors.
MATERIALS AND METHODS
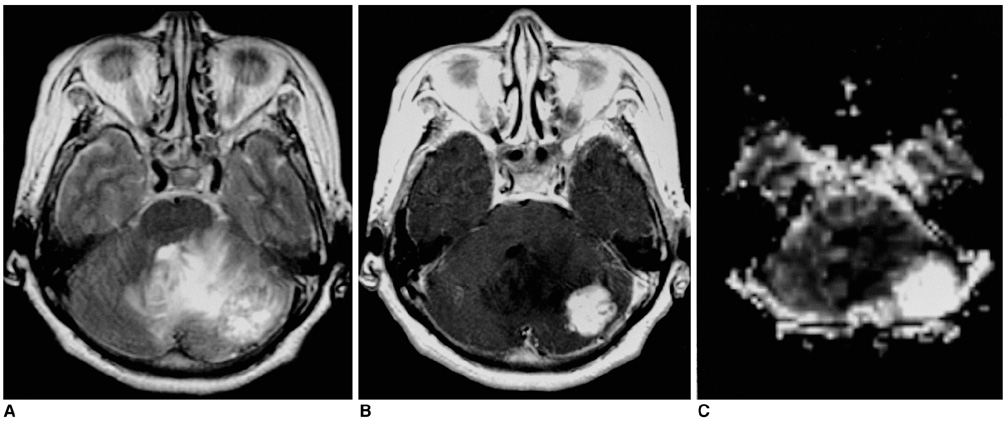
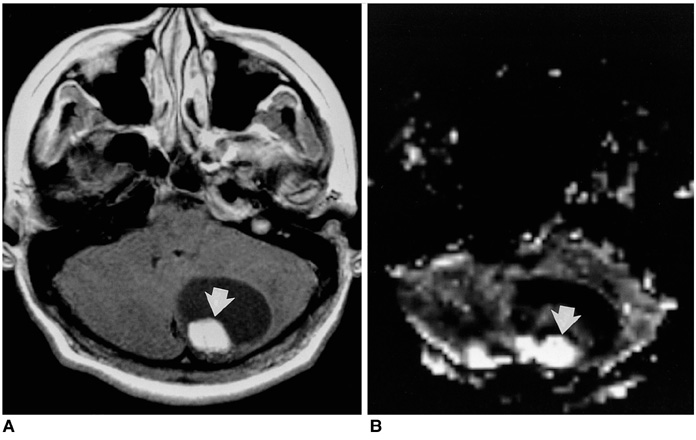
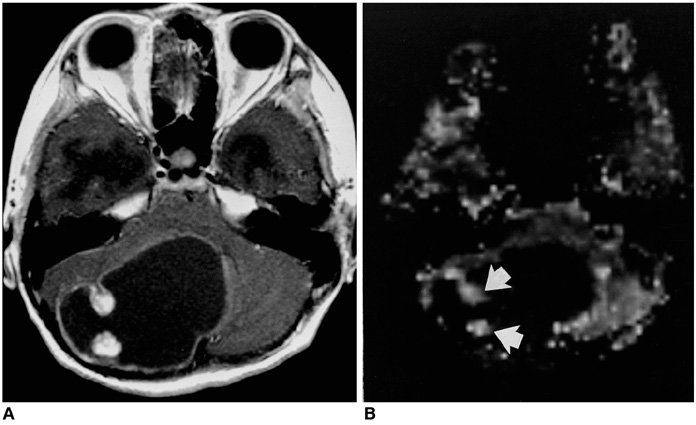
Fifty-seven patients with pathologically proven brain tumors (21 high-grade gliomas, 8 low-grade gliomas, 8 lymphomas, 6 hemangioblastomas, 7 metastases, and 7 various other tumors) were included in this study. Relative cerebral blood volume (rCBV) and time-to-peak (TTP) ratios were quantitatively analyzed and the rCBV grade of each tumor was also visually assessed on an rCBV map.
RESULTS
The highest rCBV ratios were seen in hemangioblastomas, followed by high-grade gliomas, metastases, low-grade gliomas, and lymphomas. There was no significant difference in TTP ratios between each tumor group (p<0.05). At visual assessment, rCBV was high in 17 (81%) of 21 high-grade gliomas and in 4 (50%) of 8 low-grade gliomas. Hemangioblastomas showed the highest rCBV and lymphomas the lowest.
CONCLUSION
Perfusion MR imaging may be helpful in the differentiation of thevarious solid tumors found in the brain, and in assessing the grade of the various glial tumors occurring there.
Keyword
MeSH Terms
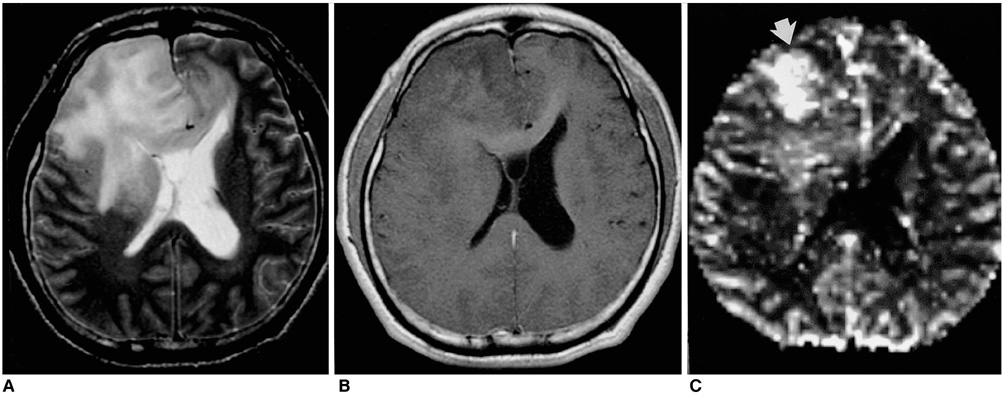
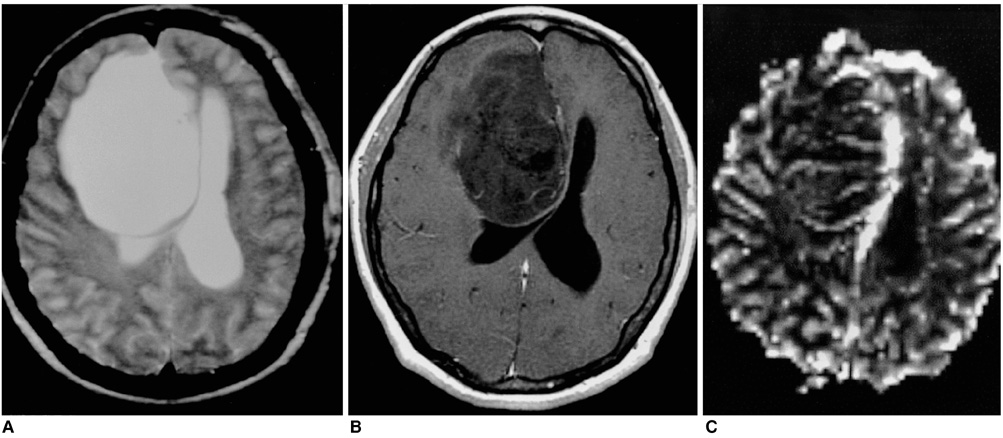
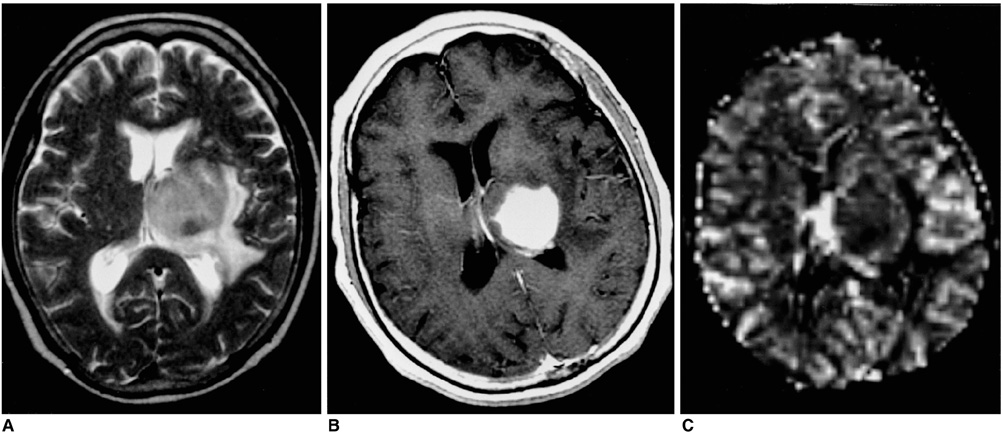
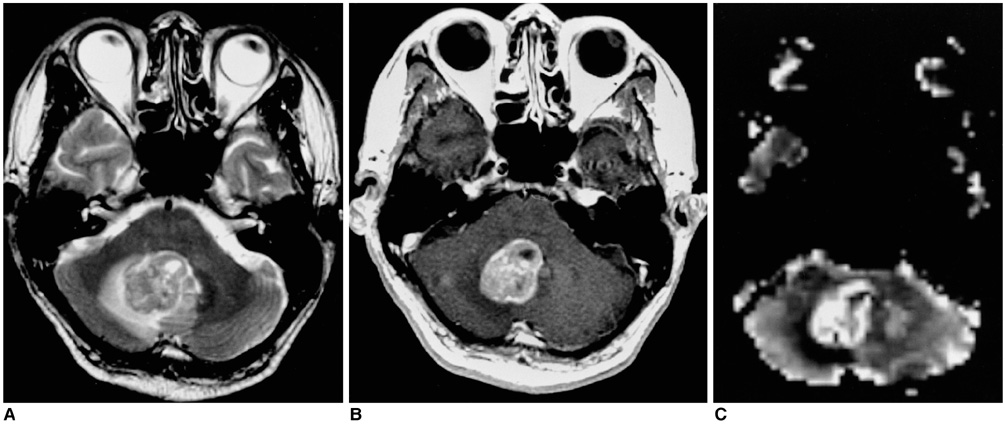
Figure
Cited by 2 articles
-
Advances in magnetic resonance technique for tumor imaging
Dong Woo Park
J Korean Med Assoc. 2015;58(6):516-522. doi: 10.5124/jkma.2015.58.6.516.Radiomics MRI Phenotyping with Machine Learning to Predict the Grade of Lower-Grade Gliomas: A Study Focused on Nonenhancing Tumors
Yae Won Park, Yoon Seong Choi, Sung Soo Ahn, Jong Hee Chang, Se Hoon Kim, Seung-Koo Lee
Korean J Radiol. 2019;20(9):1381-1389. doi: 10.3348/kjr.2018.0814.
Reference
-
1. Brunetti A, Alfano B, Soricelli A, et al. Functional characterization of brain tumors: an overview of the potential clinical value. Nucl Med Biol. 1996. 23:699–715.2. Villringer A, Rosen BR, Belliveau JW, et al. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn Reson Med. 1988. 6:164–174.3. Rosen BR, Belliveau JW, Aronen HJ, et al. Susceptibility contrast imaging of cerebral blood volume: human experience. Magn Reson Med. 1991. 22:293–299.4. Belliveau JW, Rosen BR, Kantor HL, et al. Functional cerebral imaging by susceptibility - contrast NMR. Magn Reson Med. 1990. 14:538–546.5. Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999. 211:791–798.6. Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994. 191:41–51.7. Aronen HJ, Glass J, Pardo FS, et al. Echo-planar MR cerebral blood volume mapping of gliomas: clinical utility. Acta Radiol. 1995. 36:520–528.8. Maeda M, Itoh S, Kimura H, et al. Vascularity of meningiomas and neuromas: assessment with dynamic susceptibility-contrast MR imaging. AJR. 1994. 163:181–186.9. Bagley LJ, Grossman RI, Judy KD, et al. Gliomas: correlation of magnetic susceptibility artifact with histologic grade. Radiology. 1997. 202:511–516.10. Siegal T, Rubinstein R, Tzuk-Shina T, Gomori JM. Utility of relative cerebral blood volume mapping derived from perfusion magnetic resonance imaging in the routine follow-up of brain tumors. J Neurosurg. 1997. 86:22–27.11. Wong JC, Provenzale JM, Petrella JR. Perfusion MR imaging of brain neoplasms. AJR. 2000. 174:1147–1157.12. Lee SJ, Kim JH, Kim YM, et al. Perfusion MR imaging in gliomas: comparison with histologic tumor grade. Korean J Radiol. 2001. 2:1–7.13. Russell D, Rubinstein L. Rubinstein LJ, editor. Tumours of central neuroepithelial origin. Pathology of tumours of the nervous system. 1989. Baltimore: Williams & Wilkins;83–350.14. Glantz MJ, Burger PC, Herndon JE II, et al. Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991. 41:1741–1744.15. Rosen BR, Belliveau JW, Vevea JM, et al. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990. 14:249–265.16. Weisskoff R, Belliveau J, Kwong K, Rosen B. Potchen E, editor. Functional MR imaging of capillary hemodynamics. Magnetic resonance angiography: concepts and applications. 1993. St Louis: Mosby;473–484.17. Berchtenbreiter C, Bruening R, Wu RH, et al. Comparison of the diagnostic information in relative cerebral blood volume, maximum concentration, and subtraction signal intensity maps based on magnetic resonance imaging of gliomas. Invest Radiol. 1999. 34:75–81.18. Bitzer M, Klose U, Nagele T, et al. Echo-planar perfusion imaging with high spatial and temporal resolution: methodology and clinical aspects. Eur Radiol. 1999. 9:221–229.19. Di Chiro G, Brooks R, Bairamian D. Reivich M, Alavi A, editors. Diagnostic and prognostic value of positron emission tomography using [18F] fluorodeoxyglucose in brain tumors. Positron emission tomography. 1985. New York: Liss;291–309.20. Brasch RC, Weinmann HJ, Wesbey GE. Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR. 1984. 142:625–630.21. Grossman I, Wolf G, Biery D, et al. Gadolinium-enhanced nuclear magnetic resonance images of experimental brain abscess. J Comput Assist Tomogr. 1984. 8:204–207.22. Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR. 1998. 171:1479–1486.23. Lev MH, Rosen BR. Clinical applications of intracranial perfusion MR imaging. Neuroimaging Clin N Am. 1999. 9:309–331.24. Sugahara T, Korogi Y, Shigematsu Y, et al. Perfusion-sensitive MRI of cerebral lymphomas: a preliminary report. J Comput Assist Tomogr. 1999. 23:232–237.25. Lee SR, Sanches J, Mark AS, Dillon WP, Norman D, Newton TH. Posterior fossa hemangioblastomas: MR imaging. Radiology. 1989. 171:463–468.26. Ho VB, Smirniotopoulos JG, Murphy FM, Rushing EJ. Radiologic-pathologic correlation: hemangioblastoma. AJNR. 1992. 13:1343–1352.27. Martel AL, Allder SJ, Delay GS, Morgan PS, Moody AA. Perfusion MRI of infarcted and noninfarcted brain tissue in stroke: a comparison of conventional hemodynamic imaging and factor analysis of dynamic studies. Invest Radiol. 2001. 36:378–385.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regional Cortical Hyperperfusion on Perfusion CT during Postictal Motor Deficit: A Case Report
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Modern Brain Tumor Imaging
- Emerging Techniques in Brain Tumor Imaging: What Radiologists Need to Know
- Effect of Steroid on Brain Tumors and Surround Edemas: Observa tion with Regional Cere b ral Blood Volume (rCBV) Maps of Perfusion MRI