Korean J Radiol.
2003 Mar;4(1):1-8. 10.3348/kjr.2003.4.1.1.
Detection of Small Hypervascular Hepatocellular Carcinomas in Cirrhotic Patients: Comparison of Superparamagnetic Iron Oxide-Enhanced MR Imaging with Dual-Phase Spiral CT
- Affiliations
-
- 1Department of Diagnostic Radiology, Seoul National University Hospital, Seoul, Korea. leejm@radcom.snu.ac.kr
- 2Department of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea.
- 3Department of Diagnostic Radiology, Chonbuk National University Hospital, Chonju, Korea.
- KMID: 754036
- DOI: http://doi.org/10.3348/kjr.2003.4.1.1
Abstract
OBJECTIVE
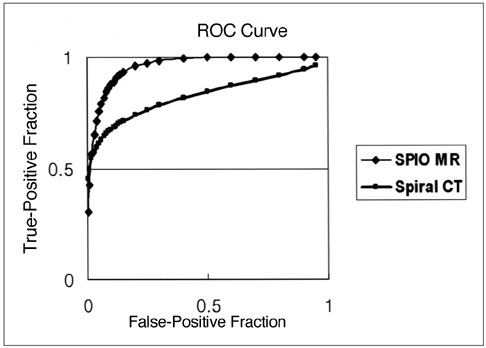
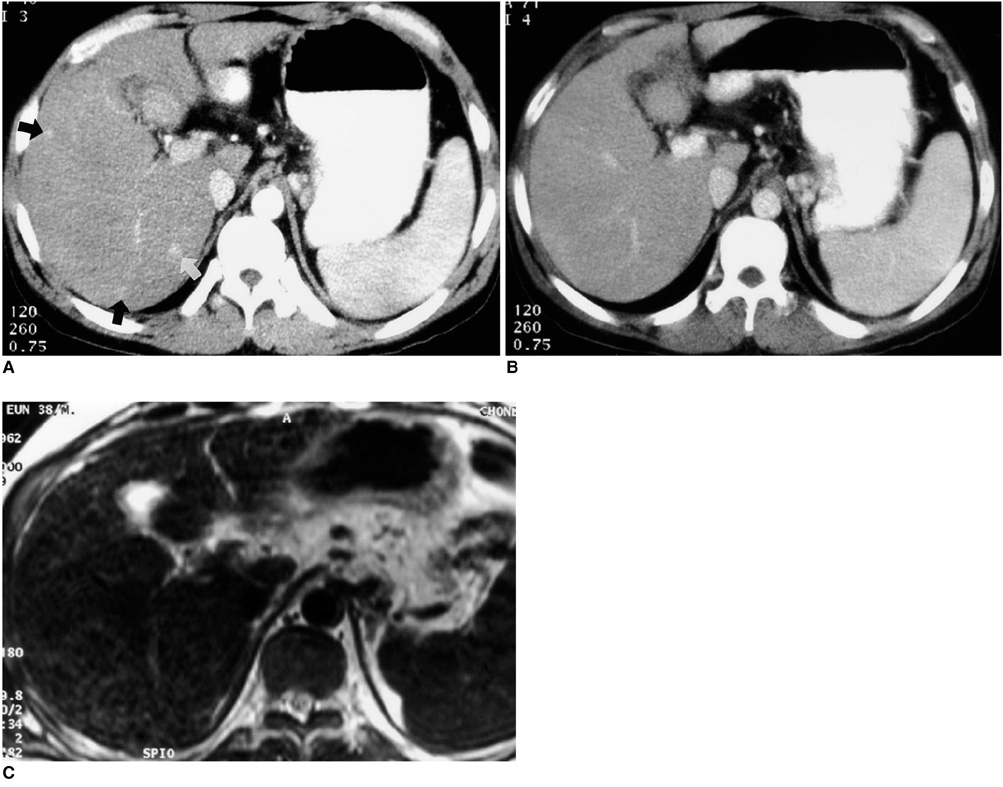
To compare the performance of superparamagnetic iron oxide (SPIO) -enhanced magnetic resonance (MR) imaging at 1.5T and dual-phase spiral computed tomography (CT) for the depiction of small hypervascular hepatocellular carcinomas (HCCs). MATERIALS AND METHODS: Forty-three patients with 70 small nodular HCCs (5-20 mm; mean, 13.7 mm) were examined. Diagnosis was based on the results of surgical biopsy in 22 patients and by the combined assessment of MR imaging, lipiodol CT, alpha feto-protein levels, and angiographic findings in 21. MR imaging consisted of respiratory-triggered turbo spin-echo T2-weighted imaging, T1-weighted fast low-angle shot, and T2* -weighted fast imaging with steady-state precession imaging before and after SPIO enhancement. CT imaging was performed with 5-mm collimation and 1: 1.4 pitch, and began 30 and 65 secs after the injection of 150 mL of contrast medium at a rate of 3 mL/sec. Two blinded observers reviewed all images independently on a segment-by-segment basis. Diagnostic accuracy was evaluated using receiver operating characteristics (ROC) analysis. RESULT: The mean areas (Az) under the ROC curves were 0.85 for SPIOenhanced MR imaging and 0.79 for dual-phase spiral CT (p < .05). The mean sensitivity of SPIO-enhanced MR imaging was significantly higher than that of CT (p < .05), i.e. 70.6% for MR imaging and 58.1% for CT. MR imaging had higher false-positive rates than dual-phase spiral CT, but the difference was not statistically significant (3.7% vs 3.3%) (p > .05). CONCLUSION: SPIO-enhanced MR imaging is more sensitive than dual-phase spiral CT for the depiction of small hypervascular hepatocellular carcinomas.
Figure
Reference
-
1. Adson MA, Weiland LH. Resection of primary solid hepatic tumors. Am J Surg. 1981. 141:18–21.2. Lim RC, Bongard FS. Hepatocellular carcinoma. Arch Surg. 1984. 119:637–642.3. Fortner JG, Kim DK, Maclean BJ, et al. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978. 188:363–369.4. Iwatsuki S, Shaw BW, Starzl TE. Experience with 150 liver resections. Ann Surg. 1983. 197:247–253.5. Kanematsu T, Takenaka L, Matsumata T, Furnita T, Sugimachi K, Inokuchi K. Limited hepatic resection effective for selected cirrhotic patients with primary liver cancer. Ann Surg. 1984. 199:51–56.6. Lee KH, Choi BI, Han JK, Jang HJ, Kim TK, Han MC. Nodular hepatocellular carcinoma: variation of tumor conspicuity on single-level dynamic scan and optimization of fixed delay times for two-phase helical CT. J Comput Assist Tomogr. 2000. 24:212–218.7. Kim T, Murakami T, Takahash S, et al. Optimal phases of dynamic CT for detecting hepatocellular carcinoma: evaluation of unenhanced and triple-phase images. Abdom Imaging. 1999. 24:473–480.8. Yamashita Y, Mitsuzaki K, Yi T, et al. Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of whole liver. Radiology. 1996. 200:79–84.9. Miller WJ, Baron RL, Dodd GD III, Federle MP. Malignancies in patients with cirrhosis: CT sensitivity and specificity in 200 consecutive transplant patients. Radiology. 1994. 193:645–650.10. Winter TC III, Freeny PC, Nghiem HV, et al. MR imaging with IV superparamagnetic iron oxide: efficacy in the detection of focal hepatic lesions. AJR Am J Roentgenol. 1993. 161:1191–1198.11. Ros PR, Freeny PC, Harms SE, et al. Hepatic MR imaging with ferumoxides: a multicenter clinical trial of its safety and efficacy in the detection of focal hepatic lesions. Radiology. 1995. 196:481–488.12. Soyer P. Will ferumoxides-enhanced MR imaging replace CT during arterial portography in the detection of hepatic metastases? Prologue to a promising future. Radiology. 1996. 200:610–611.13. Matsui O, Kadoya M, Yoshikawa J, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993. 188:79–83.14. Kubota K, Hisa N, Nashikawa T, et al. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001. 26:184–190.15. Yamamoto H, Yamashita Y, Yoshimatsu S, et al. Hepatocecullular carcinoma in cirrhotic livers: detection with unenhanced and iron oxide-enhanced MR imaging. Radiology. 1995. 195:106–112.16. Tang Y, Yamashita Y, Arakawa A, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of Gadolinium-and Ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999. 172:1547–1554.17. Choi D, Kim SH, Lim JH, et al. Preoperative detection of hepatocellular carcinoma: ferumoxides-enhanced MR imaging versus combined helical CT during arterial portography and CT hepatic arteriography. AJR Am J Roentgenol. 2001. 176:475–482.18. Hagspiel KD, Neidl KFW, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced MR imaging at 1.5-T with dynamic CT, intraoperative US and percutaneous US. Radiology. 1995. 196:471–478.19. Ward J, Naik KS, Guthrie JA, Wilson D, Robison PJ. Hepatic lesion detection: comparison of MR imaging after the administration of superparamagnetic iron oxide with dual-phase CT by using alternative free-response receiver operating characteristic analysis. Radiology. 1999. 210:459–466.20. Bluemke DA, Paulson EK, Choti MA, DeSena S, Clavien PA. Detection of hepatic lesions in candidates for surgery: comparison of ferumoxides-enhanced MR imaging and dual-phase helical CT. AJR Am J Roentgenol. 2000. 175:1653–1658.21. Seneterre E, Taourel P, Bouvier Y, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996. 200:785–792.22. Elizondo G, Weissleder T, Stark DD, et al. Hepatic cirrhosis and hepatitis: MR imaging enhanced with superparamagnetic iron oxide. Radiology. 1990. 174:797–801.23. Kim TK, Choi BI, Han JK, Chung JW, Park JH, Hand MC. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998. 208:597–603.24. Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunt: dynamic CT and MR features. Korean J Radiol. 2002. 3:1–15.25. Josephson L, Lewis J, Jacob P, Hahn PF, Stark DW. The effects of iron oxides on proton relaxivity. Magn Reson Imaging. 1988. 6:647–653.26. Ward J, Chen F, Guthrie JA, et al. Hepatic lesion detection after superparamagnetic iron oxide enhancement: comparison of five T2-weighted sequences at 1.0T by using alternative free-response receiver operating characteristic analysis. Radiology. 2000. 214:159–166.27. Schwartz LH, Seltzer SE, Tempany CM, et al. Superparamagnetic iron oxide hepatic MR imaging: efficacy and safety using conventional and fast spin-echo pulse sequences. J Magn Reson Imaging. 1995. 5:566–570.28. Kim SH, Choi DI, Lim JH, et al. Optimal pulse sequence for ferumoxides-enhanced MR imaging used in the detection of hepatocellular carcinoma: comparative study using seven pulse sequences. Korean J Radiol. 2002. 3:87–97.29. Oudkerk M, van den Heuvel AG, Wielopolski PA, Shmits PIM, Borel Rinkes IHM, Wiggers T. Hepatic lesions: detection with ferumoxides-enhanced T1-weighted MR imaging. Radiology. 1997. 203:449–456.30. Grangier C, Tourniaire J, Mentha G, et al. Enhancement of liver hemangioma on T1-weighted MR SE images by superparamagnetic iron oxide particles. J Comput Assist Tomogr. 1994. 18:888–896.31. Jang HJ, Lim JH, Lee SJ, et al. Hepatocellular carcinoma: are combined CT duing arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology. 2000. 215:373–380.32. Lim JH, Choi DI, Kim SH, et al. Detection of hepatocellular carcinoma: value of adding delayed-phase imaging to dual-phase helical CT. AJR Am J Roentgenol. 2002. 179:67–73.33. Murakami T, Kim T, Takamura M, et al. Hypervascular hepatocellular carcinoma: detection with double arterial-phase multidetector row helical CT. Radiology. 2001. 218:763–767.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detectability of Hepatocellular Carcinoma: Comparison of Gd-DT PA-Enhanced and SPIO-Enhanced MR Imaging
- Intrahepatic Extramedullary Hematopoiesis Mimicking a Hypervascular Hepatic Neoplasm on Dynamic- and SPIO-Enhanced MRI
- Comparison between Spiral CT and MR Imaging in Evaluation of Focal Hepatic Masses
- Non-hypervascular Hypointense Nodules on Hepatocyte Phase Gadoxetic Acid-Enhanced MR Images: Transformation of MR Hepatobiliary Hypointense Nodules into Hypervascular Hepatocellular Carcinomas
- Focal Nodular Hyperplasia of the Liver: Imaging Findings with Emphasis on the Findings of Superparamagnetic Iron Oxide-enhanced MR Imaging