Yonsei Med J.
2008 Jun;49(3):479-485. 10.3349/ymj.2008.49.3.479.
c-fos Expression in Bladder-Specific Spinal Neurons after Spinal Cord Injury Using Pseudorabies Virus
- Affiliations
-
- 1Department of Urology and the Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea. swhan@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Anatomy, Cheju National University College of Medicine, Cheju, Korea.
- KMID: 724265
- DOI: http://doi.org/10.3349/ymj.2008.49.3.479
Abstract
- PURPOSE
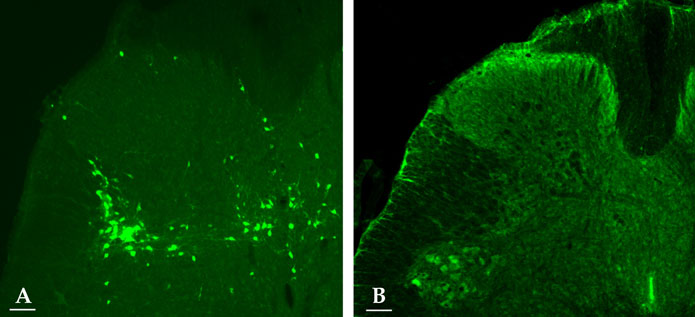
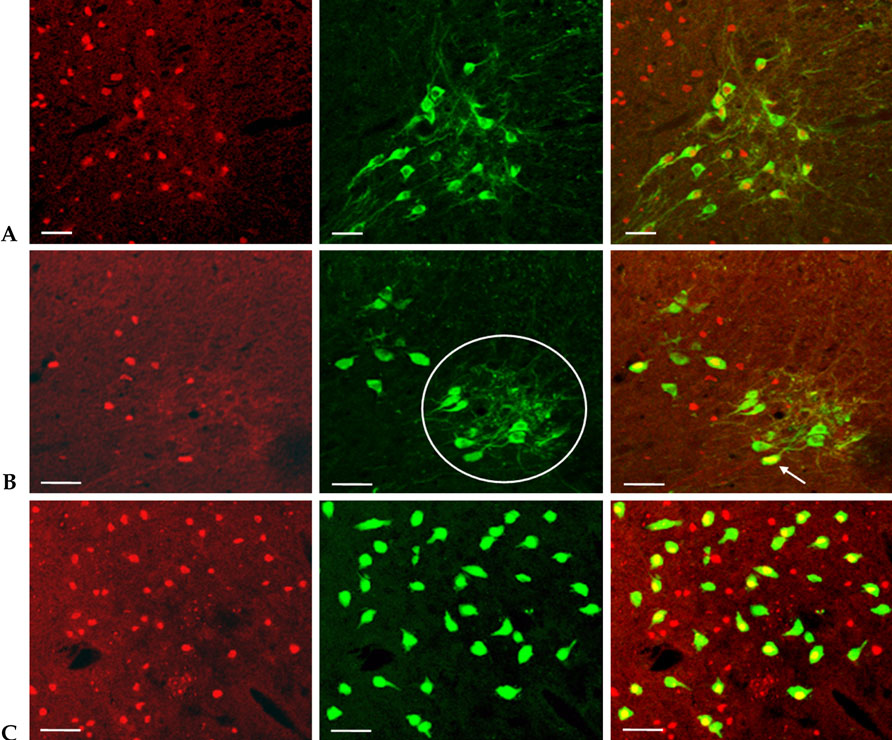
c-fos expression in spinal neurons that are activated by lower urinary tract stimulation are not organ specific. In this experiment, we demonstrated changes of c-fos expression in bladder-specific preganglionic neurons (PGNs) and interneurons using pseudorabies virus (PRV). MATERIALS AND METHODS: Forty Sprague-Dawley rats were used. We identified the neuronal pathway associated with the bladder by injecting PRV into the detrusor. An immunohistochemical method was used to stain Fos-protein encoded by the c-fos gene. Immunofluorescent staining for PRV was performed to evaluate changes in bladder-specific spinal neurons. RESULTS: Immunofluorescent staining with choline acetyltransferase (ChAT) revealed that the sacral parasympathetic nucleus (SPN) regions contained 9.8 PGNs/ section. In rats with chronic spinal cord injury by intravesical saline instillation, 82.4+/-10.3% of PGNs in SPN exhibited Fos-immunoreactive (IR). Two and a half days after PRV infection, PRV-IR PGNs were observed at 5.4 PGNs/ section, and 2.7+/-1.6% of them exhibited Fos-IR. Unlike ChAT-IR PGNs, PRV-IR PGNs are bladder-specific neurons and PRV-IR and Fos-IR cells found in the back of PRV-IR PGNs are bladder- specific interneurons. Three days after PRV infection, we observed many PRV-IR and Fos-IR cells in the dorsal commissure. These neurons are interneurons distributed in the bladder. CONCLUSION: We confirmed that in chronic spinal cord injury, the patterns of c-fos expression in bladder-specific spinal neurons were similar to those in voiding-reflex related spinal neurons, which had already been demonstrated earlier. We believe that our methodology can be applied to study interactions between voiding and other organs as well, such as the urethra and prostate.
Keyword
MeSH Terms
Figure
Reference
-
1. Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol. 1993. 264:R1157–R1163.
Article2. Vizzard MA. Alterations in growth-associated protein (GAP-43) expression in lower urinary tract pathways following chronic spinal cord injury (SCI). Somatosens Mot Res. 1999. 16:369–381.
Article3. Vizzard MA. Alternation in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol. 2000. 278:1027–1039.4. Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000. 279:R295–R305.
Article5. de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981. 3:135–160.
Article6. de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990. 30:S71–S78.
Article7. de Groat WC, Booth AM, Yoshimura N. Maggi CA, editor. Neurophysiology of micturition and its modification in animal models of human disease. Nervous control of the urogenital system: autonomic nervous system. 1993. London: Harwood Academic Publishers;227–290.8. Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci. 1992. 12:4878–4889.
Article9. Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993. 265:R326–R333.10. Hamilton MO, Papka RE, O'donoghue DL, Vaidya AM, Williams SJ, Poff CR, et al. Spinal projection neurons to the laterodorsal pontine tegmental nucleus: relationship to preganglionic neurons and nitric oxide synthase. J Comp Neurol. 1995. 353:1–8.
Article11. Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, et al. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990. 10:1974–1994.
Article12. Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, et al. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993. 13:2515–2539.
Article13. de Groat WC, Kruse MN, Vizzard MA, Cheng CL, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. Adv Neurol. 1997. 72:347–364.14. de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998. 92:127–140.
Article15. Weiss ML, Chowdhury SI. The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 1998. 812:227–241.16. Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984. 226:238–245.
Article17. Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995. 359:443–456.
Article18. Birder LA, Roppolo JR, Erickson VL, de Groat WC. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res. 1999. 834:55–65.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CNS innervation of the urinary bladder demonstrated by immunohistochemical study for c-fos and pseudorabies virus
- The Subdivision of the Spinal Neurons for Detrusor Function
- Spinal c-fos Expression in a Rat Model of Incisional Pain
- An Immunohistochemical Tracing on the Central Neural Pathways of the Spinal Accessory Nerve using Pseudorabies Virus
- Expression of Spinal c-fos in a Rat Model of Postoperative Pain