Yonsei Med J.
2008 Jun;49(3):357-365. 10.3349/ymj.2008.49.3.357.
The Influence of Type-1 Diabetes Mellitus on Dentition and Oral Health in Children and Adolescents
- Affiliations
-
- 1Department of Periodontology, Faculty of Medicine, Ataturk University, Erzurum, Turkey. receporbak@yahoo.com
- 2Department of Pedodonti, 4Endodontics, Faculty of Dentistry and 3Pediatrics, Faculty of Medicine, Ataturk University, Erzurum, Turkey.
- 3Faculty of Dentistry and Pediatrics, Faculty of Medicine, Ataturk University, Erzurum, Turkey.
- 4Department of Endodontics, Faculty of Medicine, Ataturk University, Erzurum, Turkey.
- KMID: 724249
- DOI: http://doi.org/10.3349/ymj.2008.49.3.357
Abstract
- PURPOSE
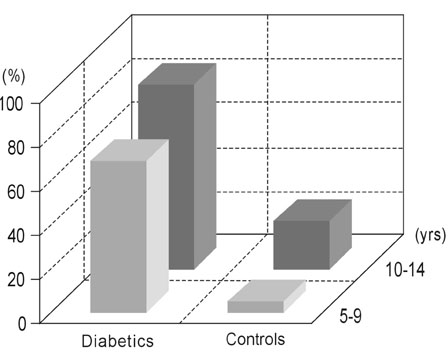
The present study is to investigate the effects of type 1 diabetes mellitus on dentition and oral health for children and adolescents. MATERIALS AND METHODS: The investigation was carried out on 100 subjects. The first group consisted of 50 subjects with type 1 diabetes mellitus (21 females, 29 males), age 9 +/- 0.14 years; In the second group, there were 50 healthy subjects who did not suffer from any systemic disease (25 females, 25 males), age 9 +/- 0.11 years. The subjects were evaluated and divided into two groups of 5-9 years old, and 10-14 years old. The dentition of all participants was examined. Besides, the DFS/dfs index, oral hygiene conditions were evaluated, as well as the plaque index (PI), gingival index (GI) and calculus index (CI). The data obtained from each group were compared statistically. RESULTS: When compared to the non-diabetic group, we observed that dental development was accelerated until the age of 10 in the diabetic group, and there was a delay after the age of 10. The edentulous interval was longer in the group with type 1 diabetes mellitus. This was accompanied by a high ratio of gingival inflammation. Gingival inflammation was 69.7% in the group of 5-9 year-old, and 83.7% in the group of 10-14 year-old with type 1 diabetes mellitus. Though there was a greater loss of teeth in the group with type 1 diabetes mellitus, there were more caries in the control group. The PI, GI and CI values showed an increase with aging in favor of the group with type 1 diabetes mellitus. There was statistically significant difference in PI, GI and CI between the control and type 1 diabetes mellitus groups for 10-14 year-old patients (p<0.001). CONCLUSION: The findings we obtained showed that type 1 diabetes mellitus plays an important part in the dentition and oral health of children and adolescents.
Keyword
MeSH Terms
Figure
Reference
-
1. Becker DJ. Lifshitz F, editor. Diabetes mellitus and hypoglycemia. Pediatric Endocrinology. 1996. 3rd ed. New York: Marcel Dekker, Inc;555–566.2. Ervasti T, Knuuttila M, Pohjamo L, Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol. 1985. 56:154–157.
Article3. Karam JH. Greenspan FS, Strewler GJ, editors. Pancreatic Hormones and diabetes mellitus. Basic and Clinical Endocrinology. 1997. 5th ed. New Jersey: Appleton & Lange;595–663.4. WHO Study Group Report. Prevention of Diabetes Mellitus. 1994. Geneva: World Health Organization;WHO Technical Report series no.844.5. Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993. 36:883–892.
Article6. Iughetti L, Marino R, Bertolani MF, Bernasconi S. Oral health in children and adolescents with IDDM-a review. J Pediatr Endocrinol Metab. 1999. 12:603–610.7. Diabetes Epidemiology Research International Group. Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes. 1988. 37:1113–1119.8. Hatun S, Tezic T. Ankaradaki okul cocuklarinda insuline bagimli diabetes mellitus prevalansi. Cocuk Sagligive Hastaliklari Dergisi. 1996. 39:465–471.9. Hanssen KF. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes. 1997. 46:Suppl 2. S101–S103.
Article10. Listgarten MA, Ricker FH Jr, Laster L, Shapiro J, Cohen DW. Vascular basement lamina thickness in the normal and inflamed gingiva of diabetics and non-diabetics. J Periodontol. 1974. 45:676–684.
Article11. Frantzis TG, Reeve CM, Brown AL Jr. The ultrastructure of capillary basement membranes in the attached gingiva of diabetics and nondiabetics patients with periodontal disease. J Periodontol. 1971. 42:406–411.
Article12. Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994. 65:260–267.
Article13. Miotti F, Ferro R, Saran G. Diabetes in oral medicine (Current status of knowledge, diagnosis, therapy and dental prevention). G Stomatol Ortognatodonzia. 1985. 4:3–14.14. Finestone AJ, Boorujy SR. Diabetes mellitus and periodontal disease. Diabetes. 1967. 16:336–340.
Article15. Sznajder N, Carraro JJ, Rugna S, Sereday M. Periodontal findings in diabetic and nondiabetics patients. J Periodontol. 1978. 49:445–448.16. Cianciola LJ, Park BH, Bruck E, Mosovich L, Genco RJ. Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent Assoc. 1982. 104:653–660.
Article17. Hugoson A, Thorstensson H, Falk H, Kuylenstierna J. Periodontal conditions in insulin-dependent diabetics. J Clin Periodontol. 1989. 16:215–223.
Article18. Benveniste R, Bixler D, Conneally PM. Periodontal disease in diabetics. J Periodontol. 1967. 38:271–279.
Article19. Hove KA, Stallard RE. Diabetes and the periodontal patient. J Periodontol. 1970. 41:713–718.
Article20. Bay I, Ainamo J, Gad T. The response of young diabetics to periodontal treatment. J Periodontol. 1974. 45:806–808.
Article21. Barnett ML, Baker RL, Yancey JM, MacMillan DR, Kotoyan M. Absence of periodontitis in a population of insulin-dependent diabetes mellitus (IDDM) patients. J Periodontol. 1984. 55:402–405.
Article22. Carranza FA, Newman MG. Irving Glickman's Clinical Periodontology Glickman. Clinical periodontology. 1996. 8th ed. Philadelphia: WB Saunders Co;281–297.23. Ziskin DE, Siegel EH, Loughlin WC. Diabetes in relation to certain oral and systemic problems. Part I: clinical study of dental caries, tooth eruption, gingival changes, growth phenomena and related observations in juveniles. J Dent Res. 1944. 23:317–331.
Article24. Bohátka L, Wegner H, Adler P. Parameters of the mixed dentition in diabetic children. J Dent Res. 1973. 52:131–135.
Article25. WHO. Oral health surveys-basic methods. 1987. 3rd ed. Geneva:26. Gröndahl HG, Hollender L, Malmcrona E, Sundquist B. Dental caries and restorations in teenagers. I. Index and score system for radiographic studies of proximal surfaces. Swed Dent J. 1997. 1:45–50.27. Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964. 24:121–135.28. Löe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand. 1963. 21:533–551.
Article29. Ramfjord SP. The periodontal disease index (PDI). J Periodontol. 1967. 38:Suppl. 602–610.
Article30. Adler P, Wegner H, Bohatka L. Influence of age and duration of diabetes on dental development in diabetic children. J Dent Res. 1973. 52:535–537.
Article31. Ben-Aryeh H, Serouya R, Kanter Y, Szargel R, Laufer D. Oral health and salivary composition in diabetic patients. J Diabetes Complications. 1993. 7:57–62.
Article32. Karjalainen KM, Knuuttila ML, Käär ML. Relationship between caries and level of metabolic balance in children and adolescents with insulin-dependent diabetes mellitus. Caries Res. 1997. 31:13–18.
Article33. Got I, Fontaine A. Teeth and diabetes. Diabete Metab. 1993. 19:467–471.34. Tchobroutsky G. Relation of diabetic control to development of microvascular complications. Diabetologia. 1978. 15:143–152.
Article35. Goteiner D, Vogel R, Deasy M, Goteiner C. Periodontal and caries experience in children with insulin-dependent diabetes mellitus. J Am Dent Assoc. 1986. 113:277–279.
Article36. Swanljung O, Meurman JH, Torkko H, Sandholm L, Kaprio E, Mäenpää J. Caries and saliva in 12-18 year old-diabetics and controls. Scand J Dent Res. 1992. 100:310–313.37. Karjalainen KM, Knuuttila ML, Käär ML. Relationship between caries and level of metabolic balance in children and adolescents with insulin-dependent diabetes mellitus. Caries Res. 1997. 31:13–18.38. Wegner H. Dental caries in young diabetics. Caries Res. 1971. 5:188–192.39. Sheridan RC Jr, Cheraskin E, Flyn AC. Epidemiology of diabetes mellitus II. 100 dental patients. J Periodontol. 1959. 30:298–323.40. van Adrichem LN, Hovius SE, van Strick R, van der Meulen JC. Acute effects of cigarette smoking on the microcirculation of the thumb. Br J Plast Surg. 1992. 45:9–11.41. Clarke NG, Shephard BC, Hirsch RS. The effects of intra-arterial epinephrine and nicotine on gingival circulation. Oral Surg Oral Med Oral Pathol. 1981. 52:577–582.
Article42. Baab DA, Oberg PA. The effect of cigarette smoking on gingival blood flow in humans. J Clin Periodontol. 1987. 14:418–424.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacothearpy of Adolescents with Diabetes
- Social Services Information for Children and Adolescents with Diabetes Mellitus
- Management of Type 2 Diabetes Mellitus in Adolescents and Young Adults
- Type 2 diabetes mellitus and metabolic syndrome
- Diagnosis and treatment of pediatric type 2 diabetes mellitus