Cancer Res Treat.
2025 Apr;57(2):519-527. 10.4143/crt.2024.652.
Second-Line Fluoropyrimidine-Based Chemotherapy in Advanced Biliary Tract Cancer: A Meta-analysis Based on Individual Patient-Level Data of Randomized Trials
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 3Division of Oncology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 4Division of Hematology and Oncology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 5Division of Hematology and Oncology, Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Korea
- 6Clinical Research Center, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University Colledge of Medicine, Seoul, Korea
- 9Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA
- 10Department of Medicine, Weill Medical College at Cornell University, New York, NY, USA
- 11Department of Medicine, Trinity College Dublin Medical School, Dublin, Ireland
- KMID: 2566868
- DOI: http://doi.org/10.4143/crt.2024.652
Abstract
- Purpose
While fluoropyrimidine-based chemotherapy regimens are recommended second-line treatment for patients with advanced biliary tract cancer (BTC), there have been no studies comparing different regimens head-to-head.
Materials and Methods
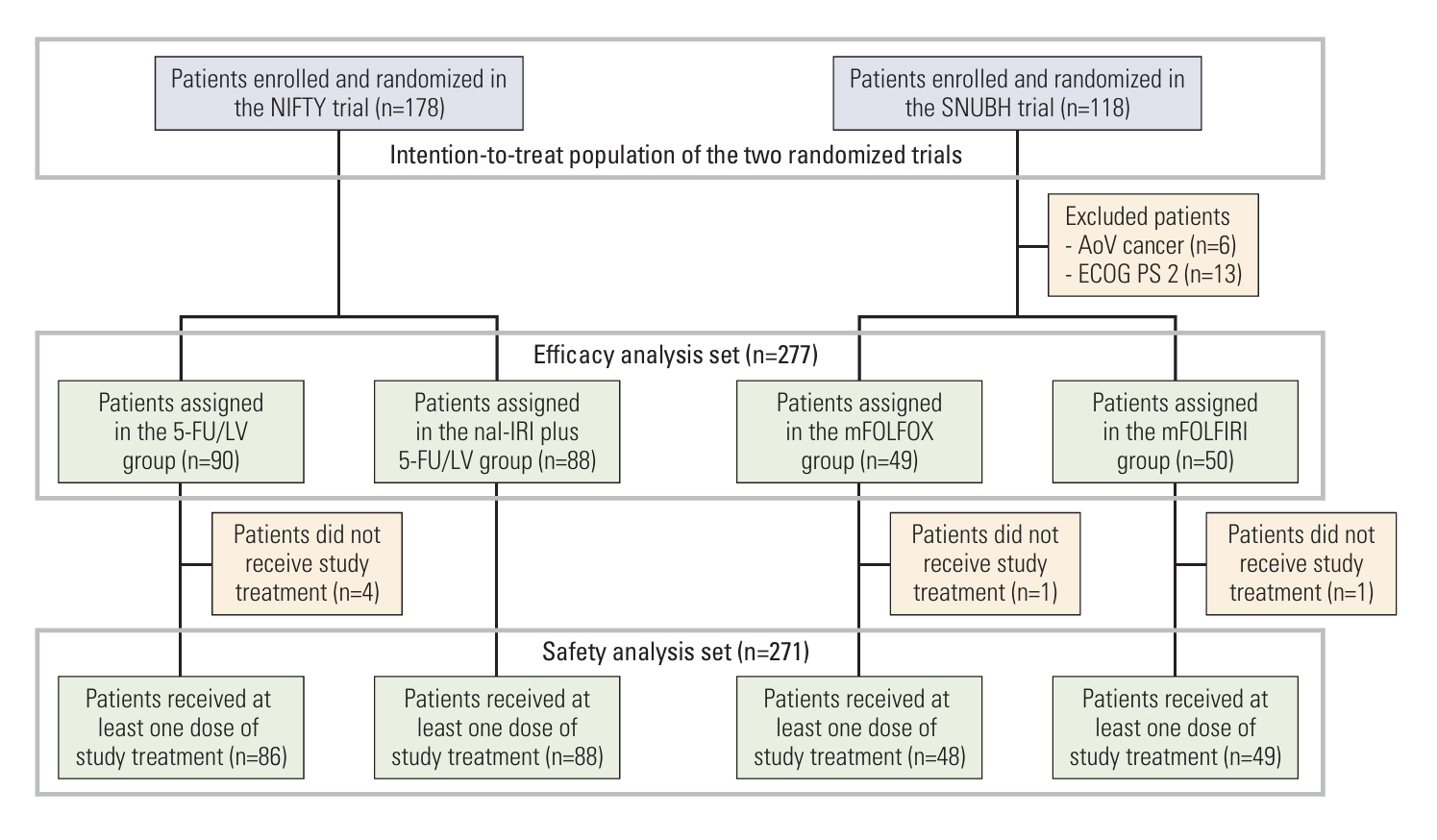
We performed individual patient-level meta-analysis based on data from the intention-to-treat population of the phase 2b NIFTY trial (liposomal irinotecan [nal-IRI] plus fluorouracil and leucovorin [5-FU/LV] vs. 5-FU/LV; NCT03542508) and the phase 2 FIReFOX trial (modified oxaliplatin plus 5-FU/LV [mFOLFOX] vs. modified irinotecan plus 5-FU/LV [mFOLFIRI]; NCT03464968). Pairwise log-rank tests and multivariable analysis using Cox proportional hazards modeling with shared frailty to account for the trial's effect were used to compare overall survival (OS) between regimens.
Results
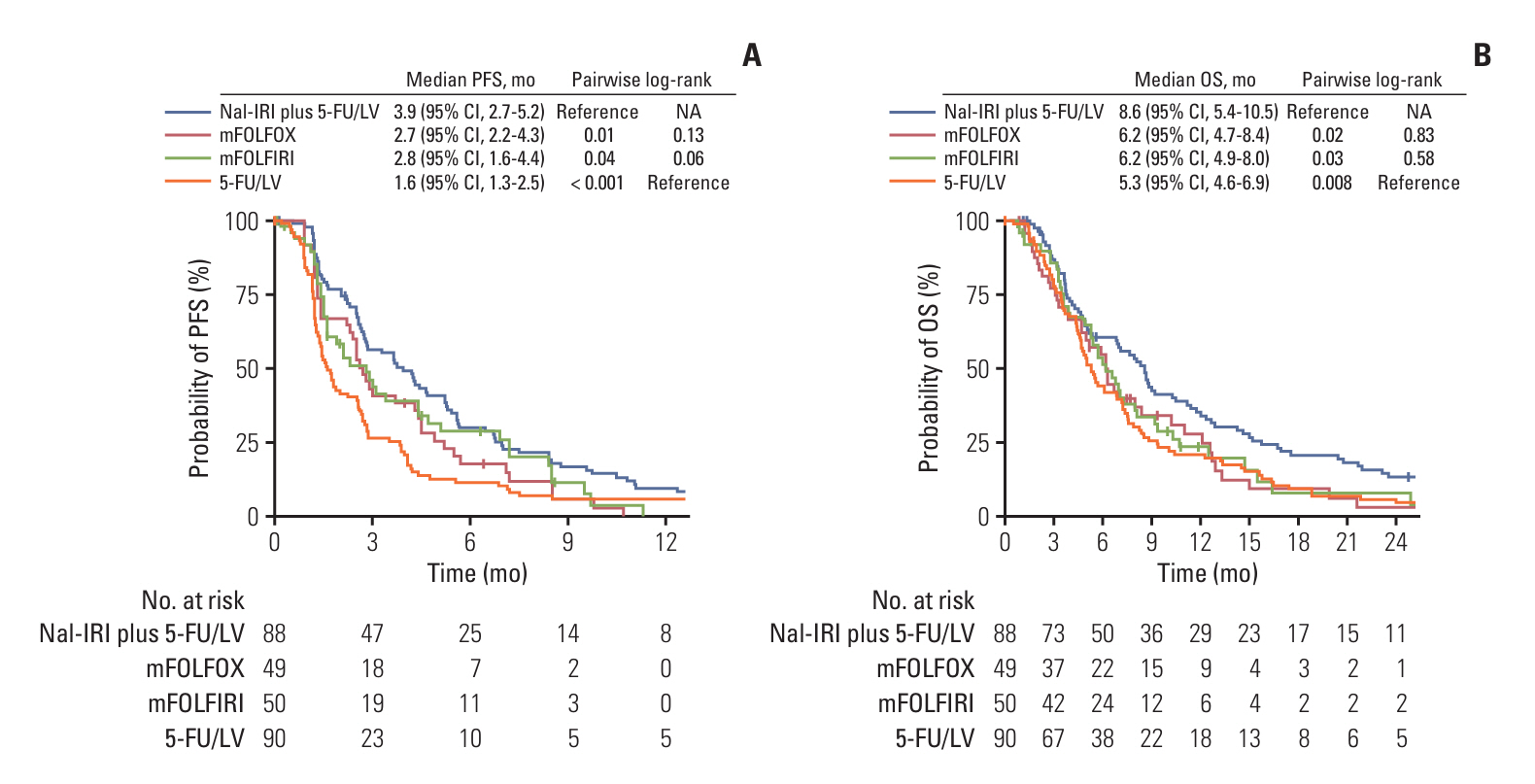
A total of 277 patients were included. The nal-IRI plus 5-FU/LV group (n=88) showed significantly better OS compared to the mFOLFOX group (n=49, pairwise log-rank, p=0.02), and mFOLFIRI group (n=50, p=0.03). Multivariable analysis showed consistent trends in OS with adjusted hazard ratios of 1.39 (mFOLFOX vs. nal-IRI plus 5-FU/LV: 95% confidence interval [CI], 0.93 to 2.07; p=0.11) and 1.36 (mFOLFIRI vs. nal-IRI plus 5-FU/LV: 95% CI, 0.92 to 2.03; p=0.13), respectively. Compared to the 5-FU/LV group, the mFOLFOX group and the mFOLFIRI group did not show differences in terms of OS (pairwise log-rank p=0.83 and p=0.58, respectively). The nal-IRI plus 5-FU/LV group experienced more frequent diarrhea, while the mFOLFOX group experienced peripheral neuropathy.
Conclusion
Nal-IRI plus 5-FU/LV showed favorable survival outcomes compared to mFOLFOX, mFOLFIRI, or 5-FU/LV. The safety profiles of these regimens should be considered along with efficacy.
Figure
Reference
-
References
1. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021; 397:428–44.
Article2. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020; 17:557–88.
Article3. Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022; 1:EVIDoa2200015.
Article4. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023; 401:1853–65.5. Vogel A, Bridgewater J, Edeline J, Kelley RK, Klumpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023; 34:127–40.
Article6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology biliary tract cancer (version 3.2023) [Internet]. National Comprehensive Cancer Network;c2023. [cited 2023 Nov 28]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf.7. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21:796–807.
Article8. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy Trial. JAMA Oncol. 2021; 7:1669–77.
Article9. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020; 21:671–84.
Article10. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021; 22:1290–300.
Article11. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N Engl J Med. 2023; 388:228–39.
Article12. Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023; 24:772–82.13. Nakamura Y, Mizuno N, Sunakawa Y, Canon JL, Galsky MD, Hamilton E, et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019): a phase II basket study. J Clin Oncol. 2023; 36:5569–78.
Article14. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021; 22:690–701.
Article15. Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021; 22:1560–72.
Article16. Hyung J, Kim I, Kim KP, Ryoo BY, Jeong JH, Kang MJ, et al. Treatment with liposomal irinotecan plus fluorouracil and leucovorin for patients with previously treated metastatic biliary tract cancer: the phase 2b NIFTY randomized clinical trial. JAMA Oncol. 2023; 9:692–9.
Article17. Choi IS, Kim KH, Lee JH, Suh KJ, Kim JW, Park JH, et al. A randomised phase II study of oxaliplatin/5-FU (mFOLFOX) versus irinotecan/5-FU (mFOLFIRI) chemotherapy in locally advanced or metastatic biliary tract cancer refractory to first-line gemcitabine/cisplatin chemotherapy. Eur J Cancer. 2021; 154:288–95.
Article18. Lowery MA, Goff LW, Keenan BP, Jordan E, Wang R, Bocobo AG, et al. Second-line chemotherapy in advanced biliary cancers: a retrospective, multicenter analysis of outcomes. Cancer. 2019; 125:4426–34.
Article19. Neuzillet C, Casadei-Gardini A, Brieau B, Vivaldi C, Brandi G, Tougeron D, et al. Fluropyrimidine single agent or doublet chemotherapy as second line treatment in advanced biliary tract cancer. Int J Cancer. 2020; 147:3177–88.
Article20. Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015; 121:3290–7.
Article21. Schweitzer N, Kirstein MM, Kratzel AM, Mederacke YS, Fischer M, Manns MP, et al. Second-line chemotherapy in biliary tract cancer: outcome and prognostic factors. Liver Int. 2019; 39:914–23.
Article22. Kim BJ, Yoo C, Kim KP, Hyung J, Park SJ, Ryoo BY, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer. 2017; 116:561–7.
Article23. Lamarca A, Hubner RA, David Ryder W, Valle JW. Secondline chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014; 25:2328–38.
Article24. Vogel A, Wenzel P, Folprecht G, Schutt P, Wege H, Kretzschmar A, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC - AIO-HEP-0116). Ann Oncol. 2022; 33(Suppl 7):S19–26.
Article25. Merz V, Messina C, Zecchetto C, Quinzii A, Frisinghelli M, Trentin C, et al. Is there room for liposomal irinotecan in biliary tract cancer? A meta-analysis of randomised trials. Clin Oncol (R Coll Radiol). 2024; 36:87–97.
Article26. Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016; 387:545–57.27. Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007; 25:1670–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Irinotecan Monotherapy Versus Irinotecan-Based Combination as Second-Line Chemotherapy in Advanced Gastric Cancer: A Meta-Analysis
- Chemotherapy for Advanced Gastric Cancer in Elderly Patients
- A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy
- Novel Palliative Chemotherapy for Cholangiocarcinoma