Cancer Res Treat.
2025 Apr;57(2):507-518. 10.4143/crt.2024.526.
Is Colonoscopy Alone Adequate for Surveillance in Stage I Colorectal Cancer?
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, Korea
- 3Department of Biopharmaceutical Convergence, Sungkyunkwan University, Seoul, Korea
- KMID: 2566867
- DOI: http://doi.org/10.4143/crt.2024.526
Abstract
- Purpose
While colonoscopy is the standard surveillance tool for stage I colorectal cancer according to National Comprehensive Cancer Network guidelines, its effectiveness in detecting recurrence is debated. This study evaluates recurrence risk factors and patterns in stage I colorectal cancer to inform comprehensive surveillance strategies.
Materials and Methods
A retrospective analysis of 2,248 stage I colorectal cancer patients who underwent radical surgery at Samsung Medical Center (2007-2018) was conducted. Exclusions were based on familial history, prior recurrences, preoperative treatments, and inadequate data. Surveillance included colonoscopy, laboratory tests, and computed tomography (CT) scans.
Results
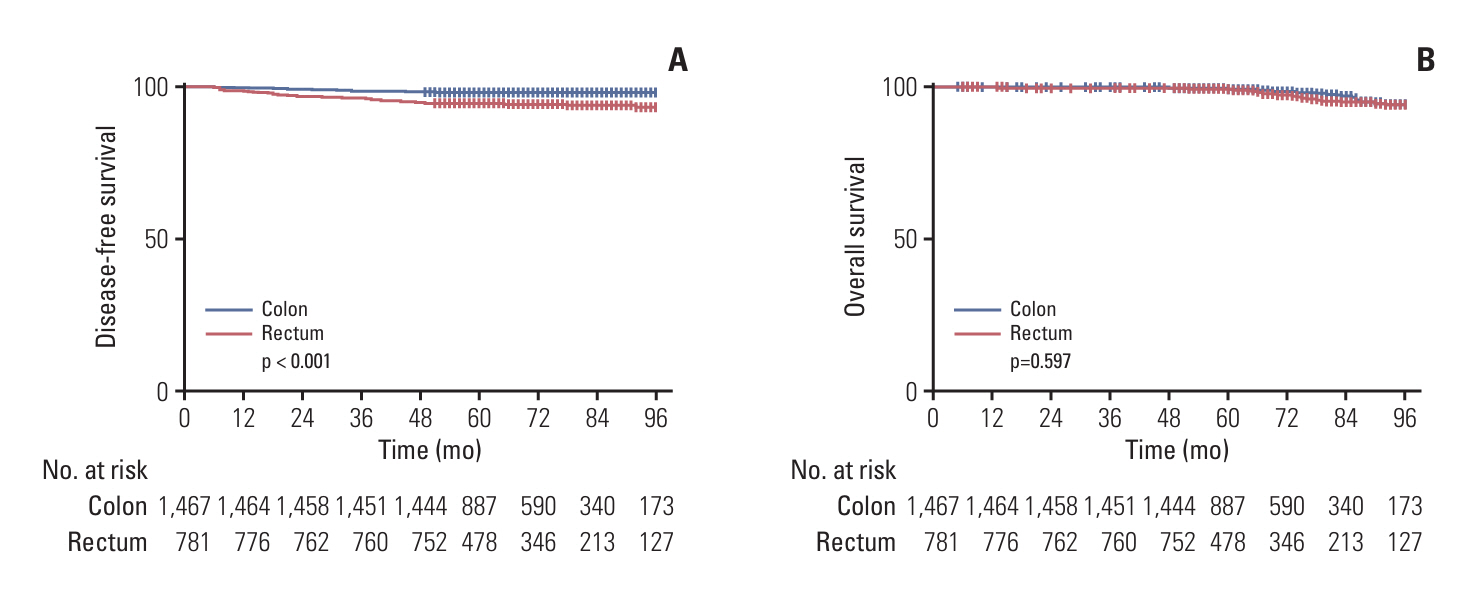
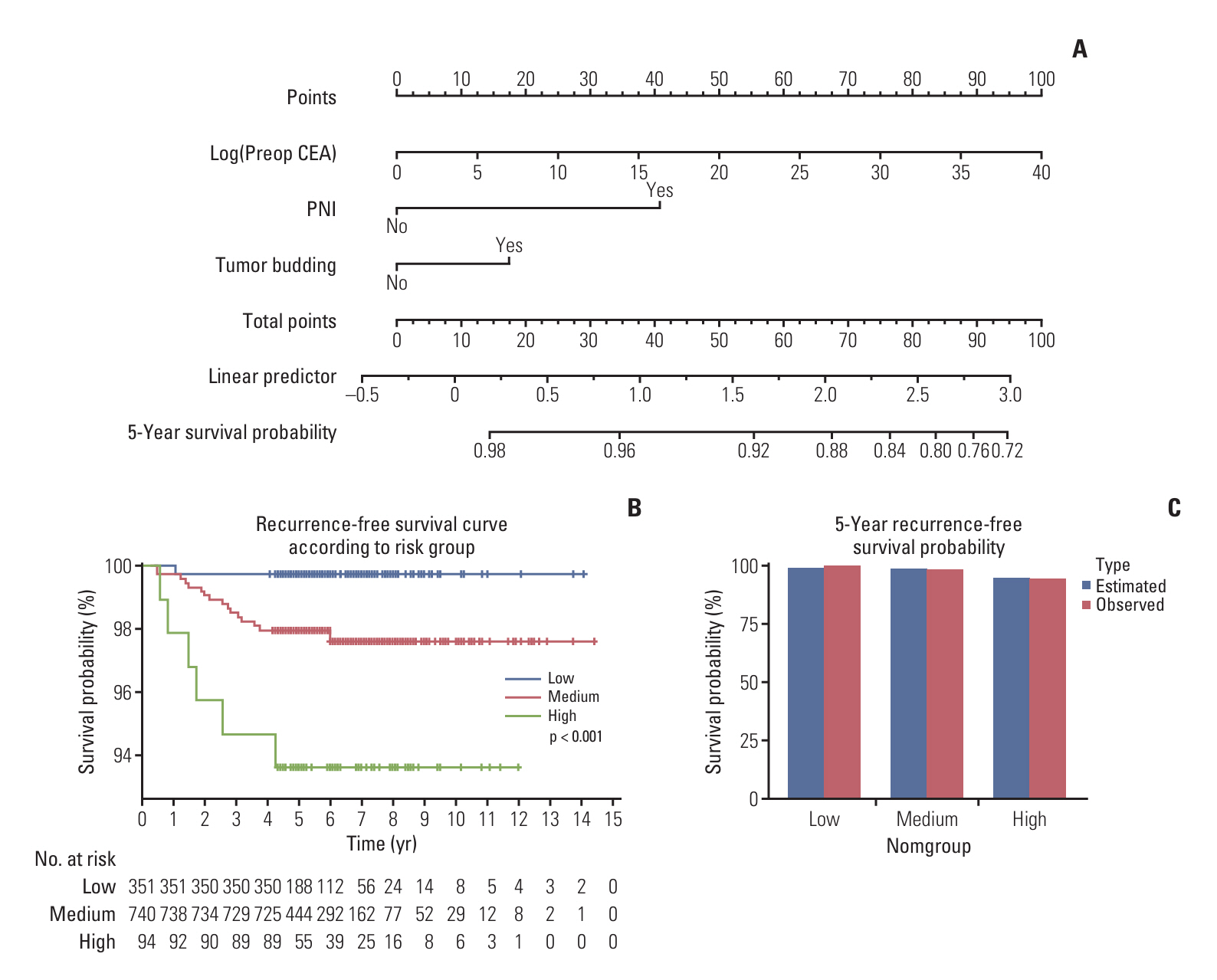
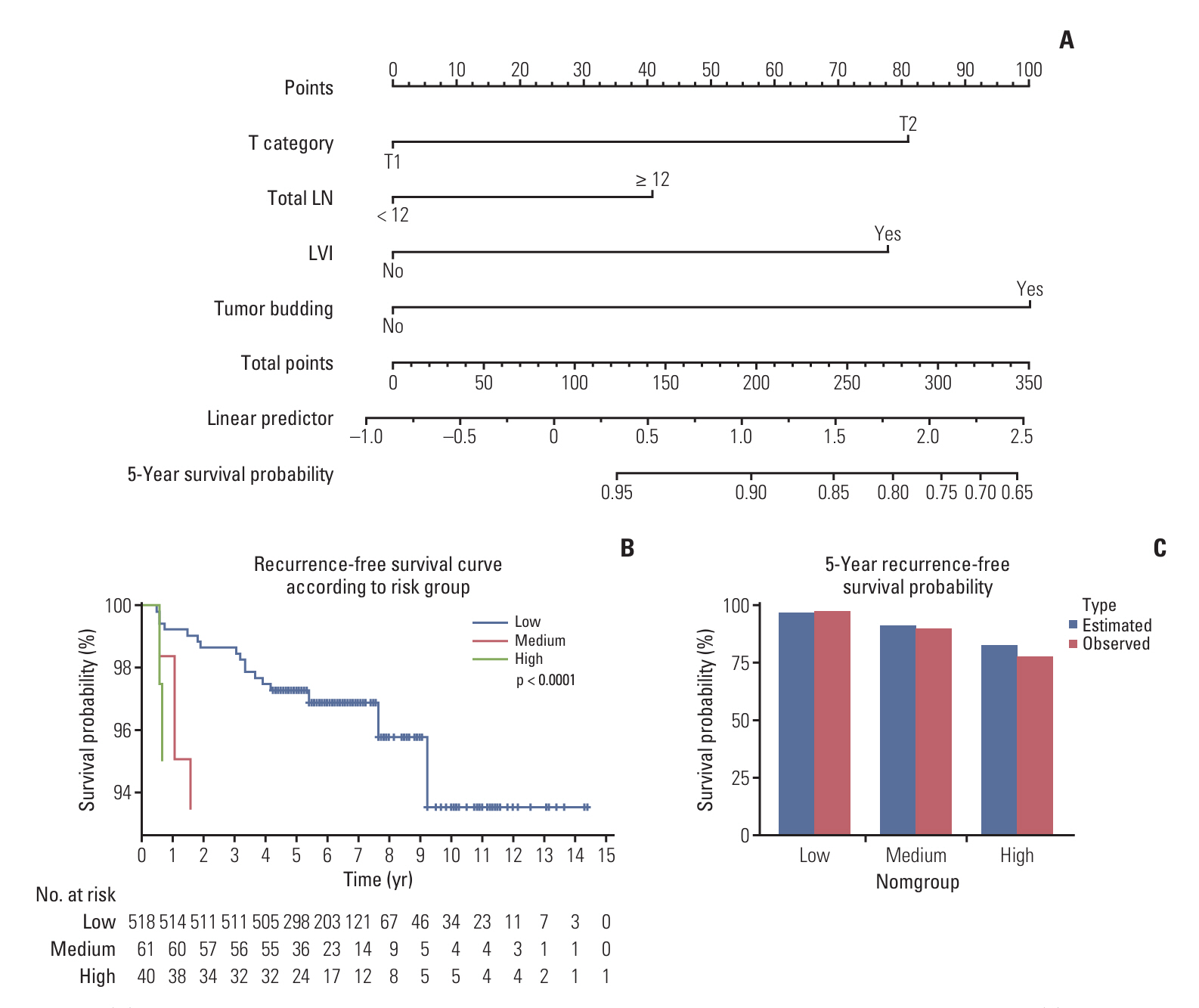
Stage I colorectal cancer patients showed favorable 5-year disease-free survival (98.3% colon, 94.6% rectum). Among a total of 1,467 colon cancer patients, 26 (1.76%) experienced recurrence. Of the 781 rectal cancer patients, 47 (6.02%) experienced recurrence. Elevated preoperative carcinoembryonic antigen levels and perineural invasion were significant recurrence risk factors in colon cancer, while tumor budding was significant in rectal cancer. Distant metastasis was the main recurrence pattern in colon cancer (92.3%), while rectal cancer showed predominantly local recurrence (50%). Colonoscopy alone detected recurrences in a small fraction of cases (3.7% in colon, 14.9% in rectum).
Conclusion
Although recurrence in stage I colorectal cancer is rare, relying solely on colonoscopy for surveillance may miss distant metastases or locoregional recurrence outside the colorectum. For high-risk patients, we recommend considering regular CT scans alongside colonoscopy. This targeted approach may enable earlier recurrence detection and improve outcomes in this subset while avoiding unnecessary scans for the low-risk majority.
Keyword
Figure
Reference
-
References
1. Li X, Zhou Y, Luo Z, Gu Y, Chen Y, Yang C, et al. The impact of screening on the survival of colorectal cancer in Shanghai, China: a population based study. BMC Public Health. 2019; 19:1016.
Article2. Cardoso R, Zhu A, Guo F, Heisser T, Hoffmeister M, Brenner H. Incidence and mortality of proximal and distal colorectal cancer in Germany: trends in the era of screening colonoscopy. Dtsch Arztebl Int. 2021; 118:281–7.3. Kim S, Huh JW, Lee WY, Yun SH, Kim HC, Cho YB, et al. Lymphovascular invasion, perineural invasion, and tumor budding are prognostic factors for stage I colon cancer recurrence. Int J Colorectal Dis. 2020; 35:881–5.
Article4. Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015; 16:161–8.
Article5. Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T, et al. Better survival in right-sided versus left-sided stage I-III colon cancer patients. BMC Cancer. 2016; 16:554.6. Sticca RP, Rodriguez-Bigas M, Penetrante RB, Petrelli NJ. Curative resection for stage I rectal cancer: natural history, prognostic factors, and recurrence patterns. Cancer Invest. 1996; 14:491–7.
Article7. Teloken PE, Ransom D, Faragher I, Jones I, Gibbs P, Platell C. Recurrence in patients with stage I colorectal cancer. ANZ J Surg. 2016; 86:49–53.
Article8. Hardiman KM, Felder SI, Friedman G, Migaly J, Paquette IM, Feingold DL, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the surveillance and survivorship care of patients after vurative treatment of volon and rectal cancer. Dis Colon Rectum. 2021; 64:517–33.9. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022; 20:1139–67.10. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29:iv263.
Article11. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. Springer;2017.12. Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012; 25:1315–25.
Article13. Baqar AR, Wilkins S, Staples M, Angus Lee CH, Oliva K, McMurrick P. The role of preoperative CEA in the management of colorectal cancer: a cohort study from two cancer centres. Int J Surg. 2019; 64:10–5.
Article14. Becerra AZ, Probst CP, Tejani MA, Aquina CT, Gonzalez MG, Hensley BJ, et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: results from the national cancer database. Ann Surg Oncol. 2016; 23:1554–61.
Article15. Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965; 121:439–62.
Article16. Cutait R, Alves VA, Lopes LC, Cutait DE, Borges JL, Singer J, et al. Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum. 1991; 34:917–20.
Article17. Takagawa R, Fujii S, Ohta M, Nagano Y, Kunisaki C, Yamagishi S, et al. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008; 15:3433–9.
Article18. Su BB, Shi H, Wan J. Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World J Gastroenterol. 2012; 18:2121–6.
Article19. Tong G, Xu W, Zhang G, Liu J, Zheng Z, Chen Y, et al. The role of tissue and serum carcinoembryonic antigen in stages I to III of colorectal cancer: a retrospective cohort study. Cancer Med. 2018; 7:5327–38.20. Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, Bijedic N, Bjelogrlic I, Gogov B, et al. Cancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinoma. Med Arch. 2013; 67:397–401.
Article21. Moertel CG, O’Fallon JR, Go VL, O’Connell MJ, Thynne GS. The preoperative carcinoembryonic antigen test in the diagnosis, staging, and prognosis of colorectal cancer. Cancer. 1986; 58:603–10.
Article22. Margalit O, Mamtani R, Yang YX, Reiss KA, Golan T, Halpern N, et al. Assessing the prognostic value of carcinoembryonic antigen levels in stage I and II colon cancer. Eur J Cancer. 2018; 94:1–5.
Article23. Cienfuegos JA, Martinez P, Baixauli J, Beorlegui C, Rosenstone S, Sola JJ, et al. Perineural Invasion is a major prognostic and predictive factor of response to adjuvant chemotherapy in stage I-II colon cancer. Ann Surg Oncol. 2017; 24:1077–84.
Article24. Desolneux G, Burtin P, Lermite E, Bergamaschi R, Hamy A, Arnaud JP. Prognostic factors in node-negative colorectal cancer: a retrospective study from a prospective database. Int J Colorectal Dis. 2010; 25:829–34.
Article25. Oh BY, Park YA, Huh JW, Yun SH, Kim HC, Chun HK, et al. Prognostic impact of tumor-budding grade in stages 1-3 colon cancer: a retrospective cohort study. Ann Surg Oncol. 2018; 25:204–11.
Article26. Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007; 141:67–75.
Article27. Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Dis Colon Rectum. 2007; 50:1204–10.
Article28. Liu M, Qu H, Bu Z, Chen D, Jiang B, Cui M, et al. Validation of the memorial Sloan-Kettering Cancer Center nomogram to predict overall survival after curative colectomy in a Chinese colon cancer population. Ann Surg Oncol. 2015; 22:3881–7.
Article29. Kuai L, Zhang Y, Luo Y, Li W, Li XD, Zhang HP, et al. Prognostic nomogram for liver metastatic colon cancer based on histological type, tumor differentiation, and tumor deposit: a TRIPOD compliant large-scale survival study. Front Oncol. 2021; 11:604882.
Article30. van Gijn W, van Stiphout R, van de Velde CJH, Valentini V, Lammering G, Gambacorta MA, et al. Nomograms to predict survival and the risk for developing local or distant recurrence in patients with rectal cancer treated with optional short-term radiotherapy. Ann Oncol. 2015; 26:928–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal Colonoscopy Surveillance Interval after Polypectomy
- Post-colonoscopy Colorectal Cancer: Causes and Prevention of Interval Colorectal Cancer
- Colorectal Cancer after Colonoscopy: Causes and Prevention Strategies
- Comparison of Colonoscopy Surveillance Outcomes Between Young and Older Colorectal Cancer Patients
- Training in Endoscopy: Colonoscopy