Cancer Res Treat.
2025 Apr;57(2):422-433. 10.4143/crt.2024.493.
Unraveling the Impact of Sarcopenia-Induced Lymphopenia on Treatment Response and Prognosis in Patients with Stage III Non–Small Cell Lung Cancer: Insights for Optimizing Chemoradiation and Immune Checkpoint Inhibitor
- Affiliations
-
- 1Department of Radiation Oncology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 3Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2566861
- DOI: http://doi.org/10.4143/crt.2024.493
Abstract
- Purpose
Sarcopenia is a poor prognostic factor in non–small cell lung cancer (NSCLC). However, its prognostic significance in patients with NSCLC receiving immune checkpoint inhibitors (ICIs) and its relationship with lymphopenia remain unclear. We aimed to investigate the prognostic role of sarcopenia and its effect on lymphocyte recovery in patients with stage III NSCLC treated with concurrent chemoradiotherapy (CCRT) followed by ICI.
Materials and Methods
We retrospectively evaluated 151 patients with stage III NSCLC who received definitive CCRT followed by maintenance ICI between January 2016 and June 2022. Sarcopenia was evaluated by measuring the skeletal muscle area at the L3 vertebra level using computed tomography scans. Lymphocyte level changes were assessed based on measurements taken before and during CCRT and at 1, 2, 3, 6, and 12 months post-CCRT completion.
Results
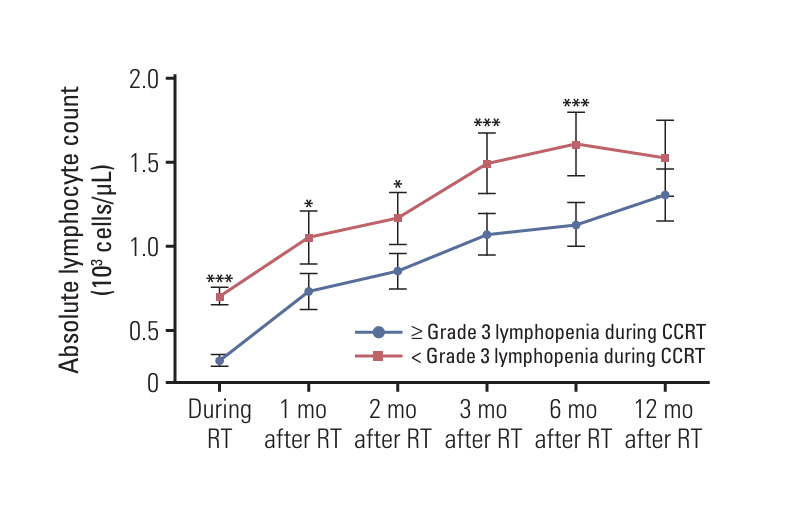
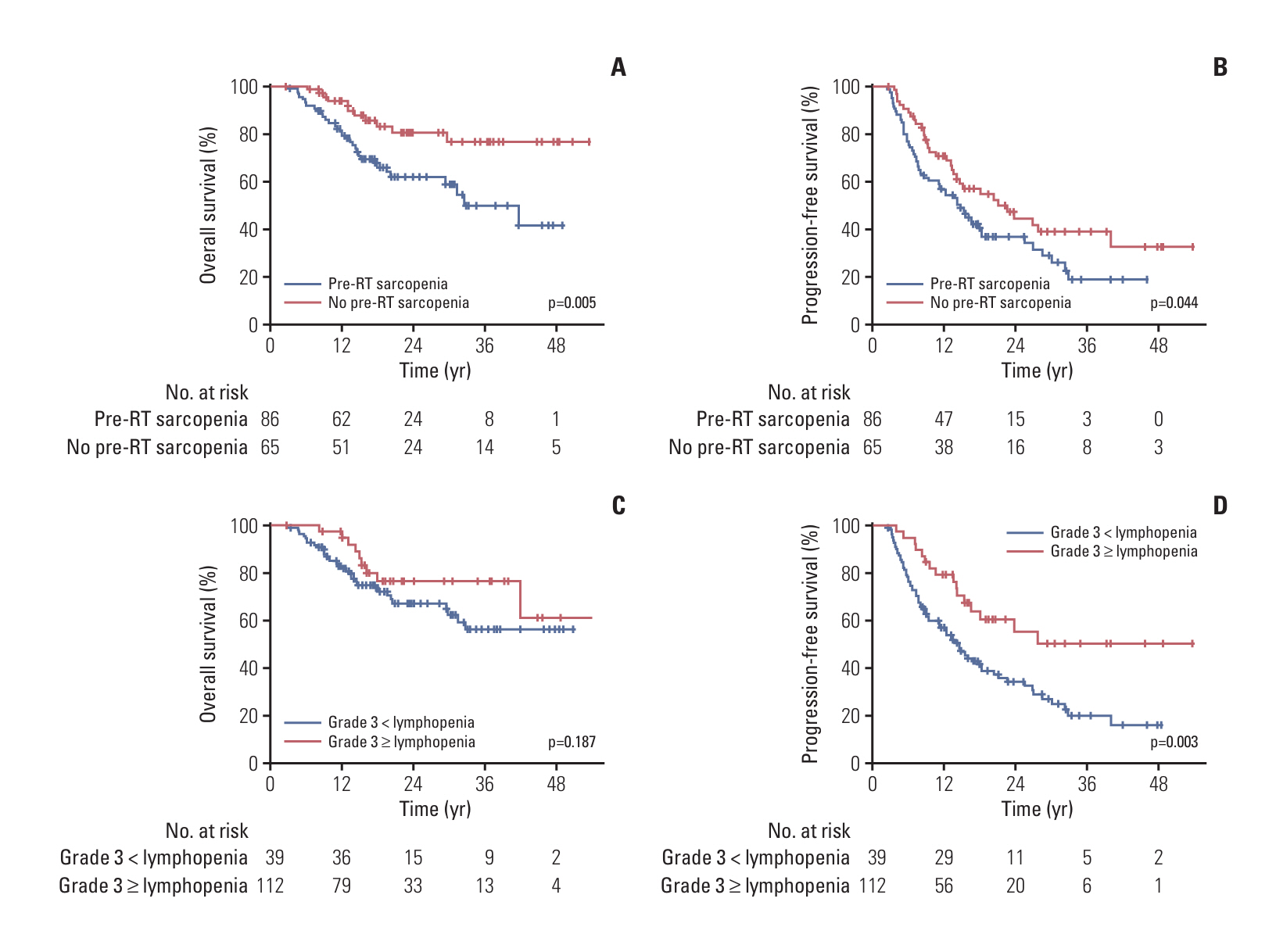
Even after adjusting for baseline absolute lymphocyte count through propensity score-matching, patients with pre-radiotherapy (RT) sarcopenia (n=86) exhibited poor lymphocyte recovery and a significantly high incidence of grade ≥ 3 lymphopenia during CCRT. Pre-RT sarcopenia and grade ≥ 3 lymphopenia during CCRT emerged as prognostic factors for overall survival and progression-free survival, respectively. Concurrent chemotherapy dose adjustments, objective response after CCRT, and discontinuation of maintenance ICI were also analyzed as independent prognostic factors.
Conclusion
Our results demonstrated an association between pre-RT sarcopenia and poor survival, concurrent chemotherapy dose adjustments, and impaired lymphocyte recovery after definitive CCRT. Moreover, CCRT-induced lymphopenia not only contributed to poor prognosis but may have also impaired the therapeutic efficacy of subsequent maintenance ICI, ultimately worsening treatment outcomes.
Keyword
Figure
Reference
-
References
1. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016; 57:58–67.
Article2. Cho Y, Kim JW, Keum KC, Lee CG, Jeung HC, Lee IJ. Prognostic significance of sarcopenia with inflammation in patients with head and neck cancer who underwent definitive chemoradiotherapy. Front Oncol. 2018; 8:457.
Article3. Lee J, Cho Y, Park S, Kim JW, Lee IJ. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with radiotherapy. Front Oncol. 2019; 9:1075.
Article4. Aspinall R. T cell development, ageing and interleukin-7. Mech Ageing Dev. 2006; 127:572–8.
Article5. Bazdar DA, Kalinowska M, Sieg SF. Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. J Infect Dis. 2009; 199:1019–28.
Article6. Haugen F, Norheim F, Lian H, Wensaas AJ, Dueland S, Berg O, et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol Cell Physiol. 2010; 298:C807–16.
Article7. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017; 377:1919–29.8. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019; 105:1065–73.
Article9. Li S, Wang T, Tong G, Li X, You D, Cong M. Prognostic impact of sarcopenia on clinical outcomes in malignancies treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol. 2021; 11:726257.
Article10. Young AC, Quach HT, Song H, Davis EJ, Moslehi JJ, Ye F, et al. Impact of body composition on outcomes from anti-PD1 +/– anti-CTLA-4 treatment in melanoma. J Immunother Cancer. 2020; 8:e000821.
Article11. Khaddour K, Gomez-Perez SL, Jain N, Patel JD, Boumber Y. Obesity, sarcopenia, and outcomes in non-small cell lung cancer patients treated with immune checkpoint inhibitors and tyrosine kinase inhibitors. Front Oncol. 2020; 10:576314.
Article12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article13. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. U.S. Department of Health and Human Services;2017.14. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998; 85:115–22.
Article15. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33:997–1006.
Article16. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12:489–95.
Article17. Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012; 67:1107–13.
Article18. Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. 2015; 10:1795–9.
Article19. Karantanos T, Karanika S, Seth B, Gignac G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clin Transl Oncol. 2019; 21:206–12.
Article20. Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014; 89:1084–91.
Article21. Friedes C, Chakrabarti T, Olson S, Prichett L, Brahmer JR, Forde PM, et al. Association of severe lymphopenia and disease progression in unresectable locally advanced non-small cell lung cancer treated with definitive chemoradiation and immunotherapy. Lung Cancer. 2021; 154:36–43.
Article22. Jing W, Xu T, Wu L, Lopez PB, Grassberger C, Ellsworth SG, et al. Severe radiation-induced lymphopenia attenuates the benefit of durvalumab after concurrent chemoradiotherapy for NSCLC. JTO Clin Res Rep. 2022; 3:100391.
Article23. Contreras JA, Lin AJ, Weiner A, Speirs C, Samson P, Mullen D, et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol. 2018; 128:498–504.
Article24. Wang X, Zhao Z, Wang P, Geng X, Zhu L, Li M. Low lymphocyte count is associated with radiotherapy parameters and affects the outcomes of esophageal squamous cell carcinoma patients. Front Oncol. 2020; 10:997.
Article25. Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol. 2017; 34:49–55.
Article26. Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018; 17:e12750.
Article27. Byun HK, Kim KJ, Han SC, Seong J. Effect of interleukin-7 on radiation-induced lymphopenia and its antitumor effects in a mouse model. Int J Radiat Oncol Biol Phys. 2021; 109:1559–69.
Article28. Lee BM, Cho Y, Kim JW, Jeung HC, Lee IJ. Prognostic significance of sarcopenia in advanced biliary tract cancer patients. Front Oncol. 2020; 10:1581.
Article29. Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest. 2019; 156:101–11.30. Wang J, Cao L, Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: a systematic review and meta-analysis. Int Immunopharmacol. 2020; 88:106907.
Article31. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022; 40:1301–11.
Article32. Cho Y, Kim Y, Chamseddine I, Lee WH, Kim HR, Lee IJ, et al. Lymphocyte dynamics during and after chemo-radiation correlate to dose and outcome in stage III NSCLC patients undergoing maintenance immunotherapy. Radiother Oncol. 2022; 168:1–7.
Article33. Kang BH, Li X, Son J, Song C, Kang HC, Kim HJ, et al. Prediction and clinical impact of delayed lymphopenia after chemoradiotherapy in locally advanced non-small cell lung cancer. Front Oncol. 2022; 12:891221.
Article34. Kuge T, Shiroyama T, Tamiya A, Tamiya M, Kanazu M, Kinehara Y, et al. Impact of lymphopenia recovery after chemoradiotherapy on durvalumab consolidation therapy in stage III NSCLC. JTO Clin Res Rep. 2023; 4:100505.
Article35. Park CK, Oh HJ, Kim YC, Kim YH, Ahn SJ, Jeong WG, et al. Korean real-world data on patients with unresectable stage III NSCLC treated with durvalumab after chemoradiotherapy: PACIFIC-KR. J Thorac Oncol. 2023; 18:1042–54.
Article36. Mooradian MJ, Cai L, Wang A, Qiao Y, Chander P, Whitaker RM. Durvalumab after chemoradiotherapy in patients with unresectable stage III non-small cell lung cancer. JAMA Netw Open. 2024; 7:e247542.
Article37. Lee DS, Kim YS, Kang JH, Lee SN, Kim YK, Ahn MI, et al. Clinical responses and prognostic indicators of concurrent chemoradiation for non-small cell lung cancer. Cancer Res Treat. 2011; 43:32–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perioperative immunotherapy in stage IB-III non-small cell lung cancer: a critical review of its rationale and considerations
- Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer
- Current advances in the treatment of lung cancer with immune checkpoint inhibitors
- Immunotherapy for Non-Small Cell Lung Cancer
- Practice guidelines for management of uterine corpus cancer in Korea: a Korean Society of Gynecologic Oncology consensus statement