Kosin Med J.
2024 Dec;39(4):254-258. 10.7180/kmj.24.143.
Efficacy of intravesical gemcitabine instillation compared with intravesical Bacillus Calmette-Guérin instillation for non-muscle invasive bladder cancer
- Affiliations
-
- 1Department of Urology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2562808
- DOI: http://doi.org/10.7180/kmj.24.143
Abstract
- Background
Intravesical Bacillus Calmette-Guérin (BCG) instillation is the most effective treatment for reducing intravesical recurrence in non-muscle invasive bladder cancer (NMIBC). However, due to the recent global shortage of BCG, there is an increasing need for alternative treatments. This study aimed to retrospectively compare the outcomes of patients treated with intravesical gemcitabine instillation and BCG instillation as initial treatment options for NMIBC.
Methods
Seventy-eight patients with NMIBC who underwent transurethral resection of bladder tumors between January 2022 and September 2023 were reviewed. Of these, 42 patients received intravesical gemcitabine instillation, and 36 patients received BCG instillation. Recurrence-free survival (RFS) was analyzed, along with tumor multiplicity, grade, T stage, size, and bladder storage time after instillation, which could influence RFS.
Results
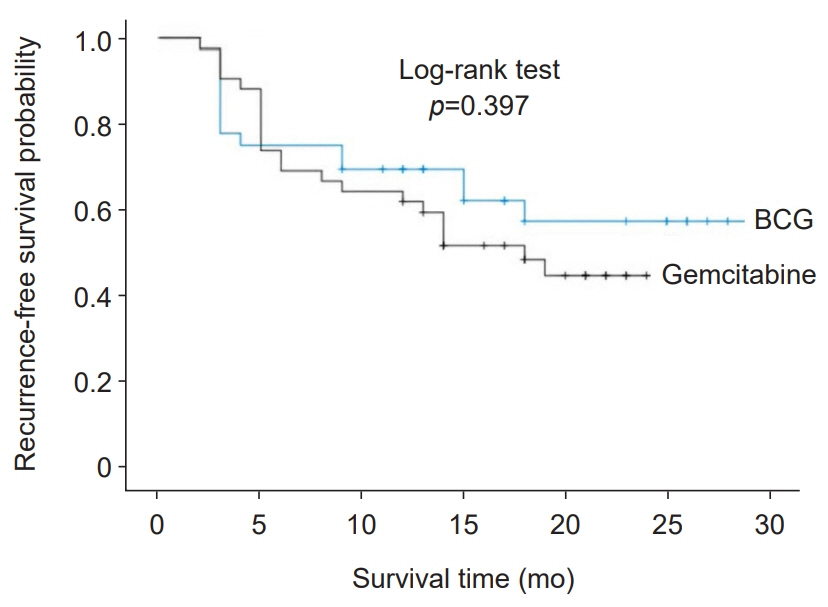
The mean follow-up period was 18.7 months for the gemcitabine group and 20.6 months for the BCG group. Recurrence occurred in 46.15% of patients (52.38% in the gemcitabine group and 38.92% in the BCG group). Tumor characteristics, including multiplicity, grade, stage, and size, were not significantly different between the two groups. The mean RFS was 15.92 months in the gemcitabine group and 19.84 months in the BCG group, with no statistically significant difference (p=0.397). However, gemcitabine instillation caused more severe bladder irritation, with shorter bladder storage time.
Conclusions
Intravesical gemcitabine and BCG instillation yielded comparable RFS outcomes. However, gemcitabine led to more severe bladder irritation, highlighting the need for further studies to optimize its application.
Figure
Reference
-
References
1. Han MA, Maisch P, Jung JH, Hwang JE, Narayan V, Cleves A, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev. 2021; 6:CD009294.
Article2. Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018; 15:615–25.
Article3. Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol. 1980; 124:38–40.
Article4. Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance Bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000; 163:1124–9.
Article5. Perera M, Papa N, Christidis D, McGrath S, Manning T, Roberts M, et al. The impact of the global bacille Calmette-Guerin shortage on treatment patterns: population-based data. BJU Int. 2018; 121:169–72.6. Balasubramanian A, Gunjur A, Weickhardt A, Papa N, Bolton D, Lawrentschuk N, et al. Adjuvant therapies for non-muscle-invasive bladder cancer: advances during BCG shortage. World J Urol. 2022; 40:1111–24.
Article7. de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014; 741:8–16.
Article8. Ye Z, Chen J, Hong Y, Xin W, Yang S, Rao Y. The efficacy and safety of intravesical gemcitabine vs bacille Calmette-Guerin for adjuvant treatment of non-muscle invasive bladder cancer: a meta-analysis. Onco Targets Ther. 2018; 11:4641–9.9. Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HJ, Lee CT, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical Bacillus Calmette-Guerin. J Urol. 2013; 190:1200–4.10. Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010; 116:1893–900.
Article11. Bendary L, Khalil S, Shahin A, Nawar N. 1655 Intravesical gemcitabine versus Bacillus Calmette-Guerin (BCG) in treatment of non-muscle invasive bladder cancer: short term comparative study. J Urol. 2011; 185(4S):e664–5.12. Shantharam G, Amin A, Pereira J, Kott O, Mueller-Leonhard C, Mega A, et al. Intravesical docetaxel for high-risk non-muscle invasive bladder cancer after Bacillus Calmette-Guerin failure. Curr Urol. 2021; 15:33–8.13. Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, Vis AN, Witte B, Newling D, et al. Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle-invasive bladder cancer: a prospective multicentre randomised study in 2243 patients. Eur Urol. 2018; 73:226–32.
Article14. Matsumura Y, Ozaki Y, Ohmori H. Intravesical adriamycin chemotherapy in bladder cancer. Cancer Chemother Pharmacol. 1983; 11 Suppl:S69–73.
Article15. Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance Bacillus Calmette-Guerin. Eur Urol. 2016; 69:60–9.16. Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013; 119:3219–27.
Article17. Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, et al. Incidence and treatment of complications of Bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992; 147:596–600.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Initial Experiences of Intravesical Gemcitabine Instillation Followed by Bacillus Calmette-Guerin(BCG) Therapy for Treating Intermediate or High Risk Patients with Superficial Bladder Cancer
- The Effect of Oral Prednisolone on Pseudo-tumor following Bacillus Calmette Guerin Intravesical Instillation
- Intravesical bacillus Calmette–Guérin-induced myopathy presenting as rhabdomyolysis: a case report
- Tuberculous Prostatic Abscess Following Intravesical Bacillus Calmette-Guerin Instillation
- Effect of Intraperitoneal and Intravesical Bacillus Calmette-Guerin on Bladder Carcinogenesis in Rats Induced by N-butyl-N-(4-hydroxybutyl) nitrosamine