J Pathol Transl Med.
2024 Nov;58(6):265-282. 10.4132/jptm.2024.10.11.
Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
- Affiliations
-
- 1Department of Anatomical Pathology, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia
- 2Human Cancer Research Center-Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 3Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2560668
- DOI: http://doi.org/10.4132/jptm.2024.10.11
Abstract
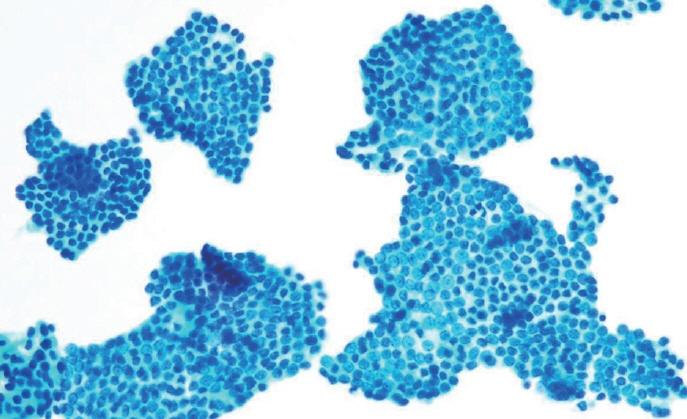
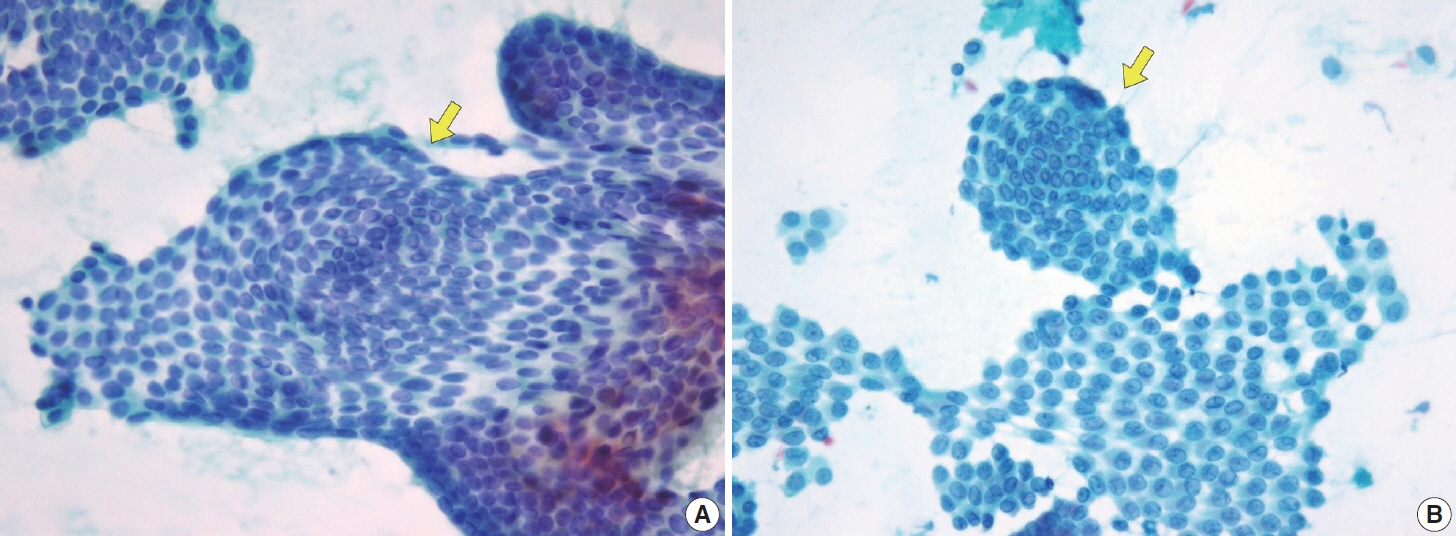
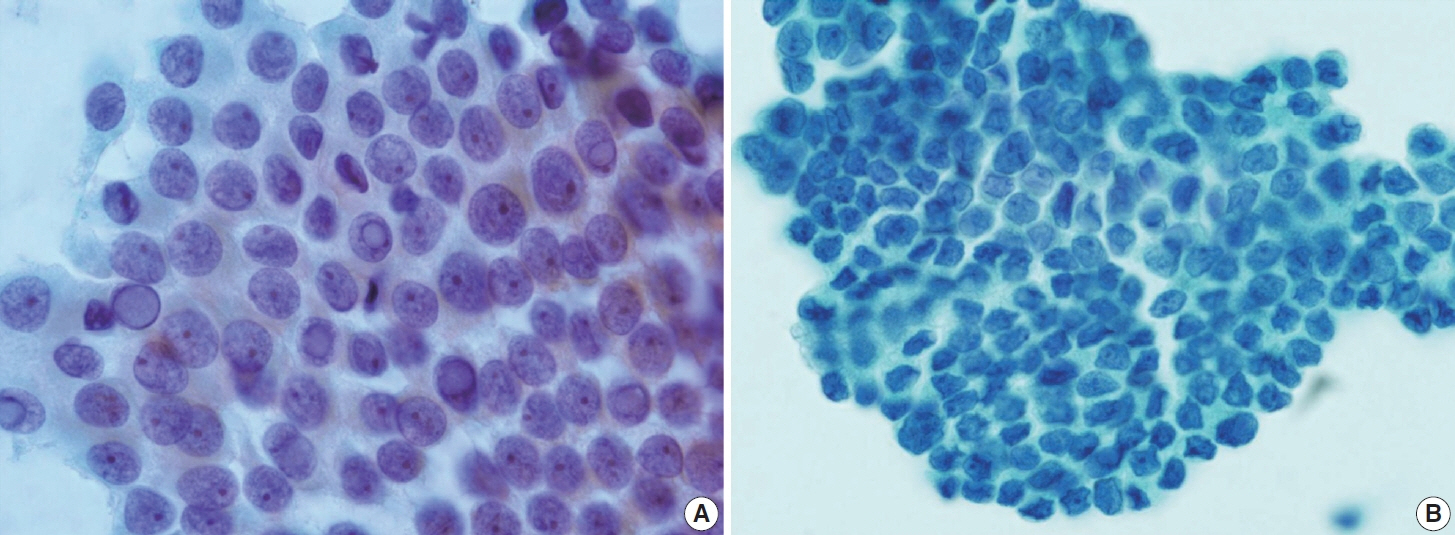
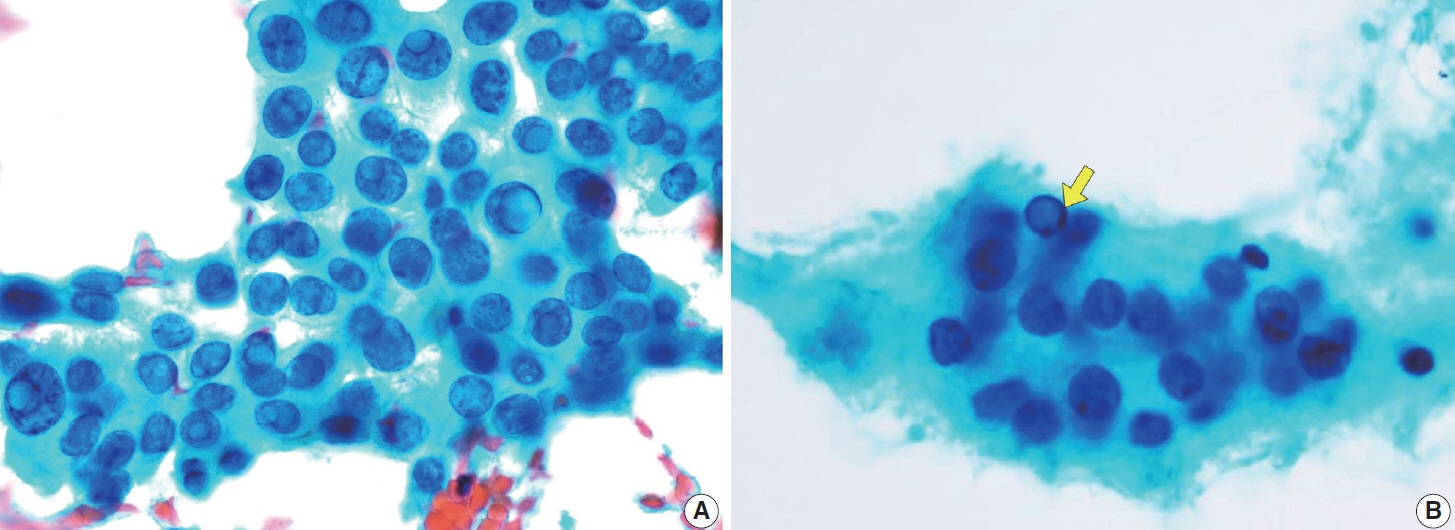
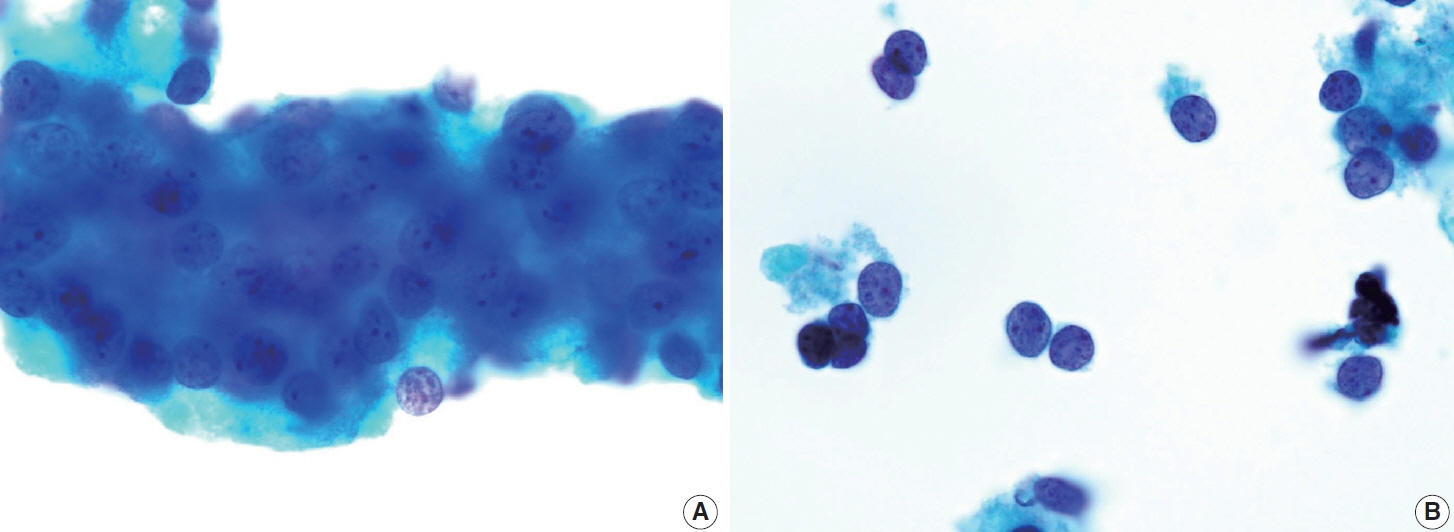
- Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, characterized by a range of subtypes that differ in their cytologic features, clinical behavior, and prognosis. Accurate cytologic evaluation of PTC using fine-needle aspiration is essential but can be challenging due to the morphologic diversity among subtypes. This review focuses on the distinct cytologic characteristics of various PTC subtypes, including the classic type, follicular variant, tall cell, columnar cell, hobnail, diffuse sclerosing, Warthin-like, solid/trabecular, and oncocytic PTCs. Each subtype demonstrates unique nuclear features, architectural patterns, and background elements essential for diagnosis and differentiation from other thyroid lesions. Recognizing these distinct cytologic patterns is essential for identifying aggressive subtypes like tall cell, hobnail, and columnar cell PTCs, which have a higher risk of recurrence, metastasis, and poorer clinical outcomes. Additionally, rare subtypes such as diffuse sclerosing and Warthin-like PTCs present unique cytologic profiles that must be carefully interpreted to avoid diagnostic errors. The review also highlights the cytologic indicators of lymph node metastasis and high-grade features, such as differentiated high-grade thyroid carcinoma. The integration of molecular testing can further refine subtype diagnosis by identifying specific genetic mutations. A thorough understanding of these subtype-specific cytologic features and molecular profiles is vital for accurate diagnosis, risk stratification, and personalized management of PTC patients. Future improvements in diagnostic techniques and standardization are needed to enhance cytologic evaluation and clinical decision-making in thyroid cancer.
Keyword
Figure
Reference
-
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024; 74:229–63.
Article2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72:7–33.
Article3. Dal Maso L, Tavilla A, Pacini F, et al. Survival of 86,690 patients with thyroid cancer: a population-based study in 29 European countries from EUROCARE-5. Eur J Cancer. 2017; 77:140–52.4. Samankan S, Militello L, Seo G, et al. Tall cell variant papillary thyroid carcinoma impacts disease-free survival at the 10 % cut-point on multivariate analysis. Pathol Res Pract. 2022; 236:154012.
Article5. Lee JS, Lee JS, Yun HJ, et al. Aggressive subtypes of papillary thyroid carcinoma smaller than 1 cm. J Clin Endocrinol Metab. 2023; 108:1370–5.
Article6. Vuong HG, Long NP, Anh NH, et al. Papillary thyroid carcinoma with tall cell features is as aggressive as tall cell variant: a meta-analysis. Endocr Connect. 2018; 7:R286–93.
Article7. Parvathareddy SK, Siraj AK, Annaiyappanaidu P, et al. Risk factors for cervical lymph node metastasis in Middle Eastern papillary thyroid microcarcinoma. J Clin Med. 2022; 11:4613.
Article8. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.9. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, Vander-Laan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol. 2023; 12:319–25.
Article10. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009; 19:1159–65.
Article11. Lee EK, Park YJ, Jung CK, Na DG. A narrative review of the 2023 Korean Thyroid Association management guideline for patients with thyroid nodules. Endocrinol Metab (Seoul). 2024; 39:61–72.
Article12. Canberk S, Montezuma D, Ince U, et al. Variants of papillary thyroid carcinoma: an algorithmic cytomorphology-based approach to cytology specimens. Acta Cytol. 2020; 64:288–98.
Article13. Rossi ED, Martini M, Capodimonti S, et al. Diagnostic and prognostic value of immunocytochemistry and BRAF mutation analysis on liquid-based biopsies of thyroid neoplasms suspicious for carcinoma. Eur J Endocrinol. 2013; 168:853–9.
Article14. Nikiforov YE, Carty SE, Chiosea SI, et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015; 25:1217–23.
Article15. Pusztaszeri M, Stelow E, Westra W, Zakowski M, Mastorakis E. Papillary thyroid carcinoma, subtypes, and related tumors. In: Ali SZ, VanderLaan PA, eds. The Bethesda System for Reporting Thyroid Cytopathology. Cham: Springer Nature;2023. p. 135–76. 3rd.16. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.
Article17. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.
Article18. Bychkov A, Jung CK. What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system. J Pathol Transl Med. 2024; 58:98–101.
Article19. Goemann IM, Romitti M, Meyer EL, Wajner SM, Maia AL. Role of thyroid hormones in the neoplastic process: an overview. Endocr Relat Cancer. 2017; 24:R367–85.
Article20. Fagin JA, Nikiforov YE. Progress in thyroid cancer genomics: a 40-year journey. Thyroid. 2023; 33:1271–86.
Article21. Nikiforov YE, Biddinger PW, Thompson LD. Diagnostic pathology and molecular genetics of the thyroid: a comprehensive guide for practicing thyroid pathology. Philadelphia: Wolters Kluwer;2020. p. 249–50. 3rd.22. Xiong XJ, Xiao MM, Zhang YX, et al. The accurate interpretation and clinical significance of morphological features of fine needle aspiration cells in papillary thyroid carcinoma. Anal Cell Pathol (Amst). 2023; 2023:9397755.
Article23. LiVolsi VA, Baloch Z. Noninvasive follicular tumor with papillary-like nuclear features: a practice changer in thyroid pathology. Arch Pathol Lab Med. 2021; 145:659–63.
Article24. Schwertheim S, Theurer S, Jastrow H, et al. New insights into intranuclear inclusions in thyroid carcinoma: association with autophagy and with BRAFV600E mutation. PLoS One. 2019; 14:e0226199.
Article25. Ashwini BR, Nirmala C, Natarajan M, Biligi DS. A study to evaluate association of nuclear grooving in benign thyroid lesions with RET/PTC1 and RET/PTC3 gene translocation. Thyroid Res. 2023; 16:21.
Article26. Henke LE, Pfeifer JD, Baranski TJ, DeWees T, Grigsby PW. Long-term outcomes of follicular variant vs classic papillary thyroid carcinoma. Endocr Connect. 2018; 7:1226–35.
Article27. Trabzonlu L, Paksoy N. Cytomorphological analysis of thyroid nodules diagnosed as follicular variant of papillary thyroid carcinoma: a fine needle aspiration study of diagnostic clues in 42 cases and the impact of using Bethesda system in reporting: an institutional experience. Endocr Pathol. 2018; 29:351–6.
Article28. Kim C, Agarwal S, Bychkov A, et al. Differentiating BRAF V600E-and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study. Virchows Arch. 2024; 484:645–56.
Article29. Yang GC, Fried KO, Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: a review of 179 cases. Diagn Cytopathol. 2017; 45:533–41.30. Basolo F, Macerola E, Poma AM, Torregrossa L. The 5(th) edition of WHO classification of tumors of endocrine organs: changes in the diagnosis of follicular-derived thyroid carcinoma. Endocrine. 2023; 80:470–6.
Article31. Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am J Clin Pathol. 2016; 146:373–7.
Article32. Yoon JH, Kwon HJ, Kim EK, Moon HJ, Kwak JY. The follicular variant of papillary thyroid carcinoma: characteristics of preoperative ultrasonography and cytology. Ultrasonography. 2016; 35:47–54.
Article33. Maleki S, Zandvakili A, Gera S, Khutti SD, Gersten A, Khader SN. Differentiating noninvasive follicular thyroid neoplasm with papillary-like nuclear features from classic papillary thyroid carcinoma: analysis of cytomorphologic descriptions using a novel machine-learning approach. J Pathol Inform. 2019; 10:29.
Article34. Haaga E, Kalfert D, Ludvikova M, Kholova I. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features is not a cytological diagnosis, but it influences cytological diagnosis outcomes: a systematic review and meta-analysis. Acta Cytol. 2022; 66:85–105.
Article35. Na HY, Park SY. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy. J Pathol Transl Med. 2022; 56:319–25.
Article36. Sohn H, Kakudo K, Jung CK. Diagnostic implication of thyroid spherules for cytological diagnosis of thyroid nodules. Cytopathology. 2024; 35:383–9.
Article37. Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ, Kang CS. Liquid-based cytology improves preoperative diagnostic accuracy of the tall cell variant of papillary thyroid carcinoma. Diagn Cytopathol. 2014; 42:11–7.
Article38. Bongiovanni M, Mermod M, Canberk S, et al. Columnar cell variant of papillary thyroid carcinoma: cytomorphological characteristics of 11 cases with histological correlation and literature review. Cancer Cytopathol. 2017; 125:389–97.
Article39. Wenig BM, Thompson LD, Adair CF, Shmookler B, Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998; 82:740–53.40. Silver CE, Owen RP, Rodrigo JP, Rinaldo A, Devaney KO, Ferlito A. Aggressive variants of papillary thyroid carcinoma. Head Neck. 2011; 33:1052–9.
Article41. Kakudo K, Liu Z, Jung CK, Hirokawa M, Bychkov A, Lai CR. Thyroid FNA cytology: differential diagnoses and pitfalls. Singapore: Springer Singapore;2023. p. 149–427. 3rd.42. Janovitz T, Williamson DF, Wong KS, Dong F, Barletta JA. Genomic profile of columnar cell variant of papillary thyroid carcinoma. Histopathology. 2021; 79:491–8.
Article43. Higgins KE, Sadow PM, Johnson DN, Wang P, Wanjari P, Cipriani NA. Columnar cell thyroid carcinoma: a heterogeneous entity demonstrating overlap between papillary thyroid carcinoma and follicular neoplasms. Head Neck Pathol. 2024; 18:39.
Article44. Ambrosi F, Righi A, Ricci C, Erickson LA, Lloyd RV, Asioli S. Hobnail variant of papillary thyroid carcinoma: a literature review. Endocr Pathol. 2017; 28:293–301.
Article45. Bellevicine C, Cozzolino I, Malapelle U, Zeppa P, Troncone G. Cytological and molecular features of papillary thyroid carcinoma with prominent hobnail features: a case report. Acta Cytol. 2012; 56:560–4.
Article46. Wong KS, Chen TY, Higgins SE, et al. A potential diagnostic pitfall for hobnail variant of papillary thyroid carcinoma. Histopathology. 2020; 76:707–13.
Article47. Malandrino P, Russo M, Regalbuto C, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016; 26:1285–92.
Article48. Pillai S, Gopalan V, Smith RA, Lam AK. Diffuse sclerosing variant of papillary thyroid carcinoma: an update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol. 2015; 94:64–73.
Article49. Cavaco D, Martins AF, Cabrera R, Vilar H, Leite V. Diffuse sclerosing variant of papillary thyroid carcinoma: outcomes of 33 cases. Eur Thyroid J. 2022; 11:e210020.
Article50. Kim SY, Shin SJ, Lee DG, et al. Clinicopathological and genetic characteristics of patients of different ages with diffuse sclerosing variant papillary thyroid carcinoma. Cancers. 2023; 15:3101.
Article51. Li W, Wang Y, Gao L, et al. Sonographic characteristics of diffuse sclerosing variant of papillary thyroid carcinoma with histopathological correlation: a preliminary study. Orphanet J Rare Dis. 2024; 19:136.
Article52. Takagi N, Hirokawa M, Nobuoka Y, Higuchi M, Kuma S, Miyauchi A. Diffuse sclerosing variant of papillary thyroid carcinoma: a study of fine needle aspiration cytology in 20 patients. Cytopathology. 2014; 25:199–204.
Article53. Houas J, Ghammam M, Chouchane L, et al. An unusual presentation of diffuse sclerosing variant of papillary thyroid carcinoma. Egypt J Otolaryngol. 2022; 38:78.
Article54. Kim J, Lim BJ, Hong SW, Pyo JY. Preoperative cytologic diagnosis of Warthin-like variant of papillary thyroid carcinoma. J Pathol Transl Med. 2018; 52:105–9.
Article55. Kalantri SH, D’Cruze L, Barathi G, Singh BK. Warthin-like papillary carcinoma thyroid. J Cancer Res Ther. 2023; 19:1471–3.
Article56. Hryshchyshyn A, Bahrii A, Botsun P, Chuba V. Warthin-like variant of papillary thyroid carcinoma with lymph node metastases: a case report and review of the literature. J Med Case Rep. 2024; 18:17.57. Chong Y, Suh S, Kim TJ, Lee EJ. Fine needle aspiration cytology of Warthin-like papillary thyroid carcinoma: a brief case report. Korean J Pathol. 2014; 48:170–3.
Article58. Ohashi R. Solid variant of papillary thyroid carcinoma: an underrecognized entity. Endocr J. 2020; 67:241–8.
Article59. Guleria P, Phulware R, Agarwal S, et al. Cytopathology of solid variant of papillary thyroid carcinoma: differential diagnoses with other thyroid tumors. Acta Cytol. 2018; 62:371–9.
Article60. Pinheiro SL, Miranda Afonso P, Damasio IL, Simoes-Pereira J, Nunes da Silva T, Leite V. Clinical significance of papillary thyroid carcinoma with solid/trabecular growth. Clin Endocrinol (Oxf). 2023; 99:335–41.
Article61. Herrera MF, Hay ID, Wu PS, et al. Hurthle cell (oxyphilic) papillary thyroid carcinoma: a variant with more aggressive biologic behavior. World J Surg. 1992; 16:669–74.62. Lukovic J, Petrovic I, Liu Z, et al. Oncocytic papillary thyroid carcinoma and oncocytic poorly differentiated thyroid carcinoma: clinical features, uptake, and response to radioactive iodine therapy, and outcome. Front Endocrinol (Lausanne). 2021; 12:795184.
Article63. Ray A, Mahore SD. An oncocytic variant of papillary thyroid carcinoma mimicking as metastatic adenocarcinoma: a diagnostic challenge. Cureus. 2023; 15:e48425.
Article64. Patnaik N, Diwaker P, Varughese AS, Arora VK, Singh B. Cytomorphological features of oncocytic variant of papillary thyroid carcinoma with lymphocytic thyroiditis. Asian J Oncol. 2016; 2:85–7.
Article65. Li G, Lei J, Peng Q, et al. Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: a single-center analysis. Medicine (Baltimore). 2017; 96:e7143.66. Masui T, Adachi S, Uemura H, Kimura T, Kitahara T. Risk factors for the lateral cervical lymph node metastasis of papillary thyroid carcinoma: a clinical study. Mol Clin Oncol. 2023; 18:25.
Article67. Liu Y, Wang Y, Zhao K, et al. Lymph node metastasis in young and middle-aged papillary thyroid carcinoma patients: a SEER-based cohort study. BMC Cancer. 2020; 20:181.
Article68. Awny S, Abdallah A, Metwally IH, et al. Impact of age on central lymph nodes involvement in papillary thyroid cancer. BMC Cancer. 2024; 24:423.
Article69. Wang P, Dong Z, Zhao S, et al. Trends of the prevalence rate of central lymph node metastasis and multifocality in patients with low-risk papillary thyroid carcinoma after delayed thyroid surgery. Front Endocrinol (Lausanne). 2024; 15:1349272.
Article70. Choi JE, Bae JS, Lim DJ, Jung SL, Jung CK. Atypical histiocytoid cells and multinucleated giant cells in fine-needle aspiration cytology of the thyroid predict lymph node metastasis of papillary thyroid varcinoma. Cancers (Basel). 2019; 11:816.
Article71. Tseng FY, Hsiao YL, Chang TC. Cytologic features of metastatic papillary thyroid carcinoma in cervical lymph nodes. Acta Cytol. 2002; 46:1043–8.
Article72. Chang YC, Lo WC, Lo CY, Liao LJ. Occult papillary thyroid carcinoma initially presenting as cervical cystic lymph node metastasis: report of two cases. J Med Ultrasound. 2013; 21:92–6.
Article73. Ng JKM, Chan ABW, Li JJX. Colloid and pigmented histiocytes in lymph node aspirates as a clue to metastasis in patients with a history of papillary thyroid carcinoma. Diagn Cytopathol. 2024; 52:22–9.
Article74. Rath A, Prabhala S, Somalwar SB, Pradeep I, Singh NK. Solid/trabecular subtype of papillary thyroid carcinoma on cytology with focal differentiated high-grade thyroid carcinoma on histology: a cyto-histologic correlation. Ecancermedicalscience. 2023; 17:1587.
Article75. Tondi Resta I, Gubbiotti MA, Montone KT, Livolsi VA, Baloch ZW. Differentiated high grade thyroid carcinomas: diagnostic consideration and clinical features. Hum Pathol. 2024; 144:53–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasound Findings of Diffuse Sclerosing Subtype of Papillary Thyroid Carcinoma
- Diagnostic Dilemma of a Follicular Lesions/Neoplasm in Thyroid Fine Needle Aspiration Cytology
- A Case of Concurrent Papillary and Medullary Thyroid Carcinomas Detected as Recurrent Medullary Carcinoma after Initial Surgery for Papillary Carcinoma
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- Medullary and Papillary Thyroid Carcinoma as a Collision Tumor: Report of Five Cases