Ann Clin Neurophysiol.
2024;26(2):46-53. 10.14253/acn.24007.
Investigation of the transcallosal ventral premotor cortex connection in humans using transcranial magnetic stimulation
- Affiliations
-
- 1Department of Neurology, Dongguk University Ilsan Hospital, Goyang, Korea
- 2Human Motor Control Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
- 3Division of Neurology, Department of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 4Clinical Neuroscience Program, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
- KMID: 2560431
- DOI: http://doi.org/10.14253/acn.24007
Abstract
- Background
The premotor cortex plays a role in the planning of movement. Previous transcranial magnetic stimulation (TMS) studies have shown ipsilateral premotor-to-motor inhibition in healthy subjects at rest. Moreover, this premotor-to-motor inhibition has been found to be modulated during preparation for movement, such as precision grip and whole hand grasp. Cooperation between the bilateral ventral premotor cortices may play a functional role. We aimed to investigate the influence of the contralateral on the ipsilateral ventral premotor cortex.
Methods
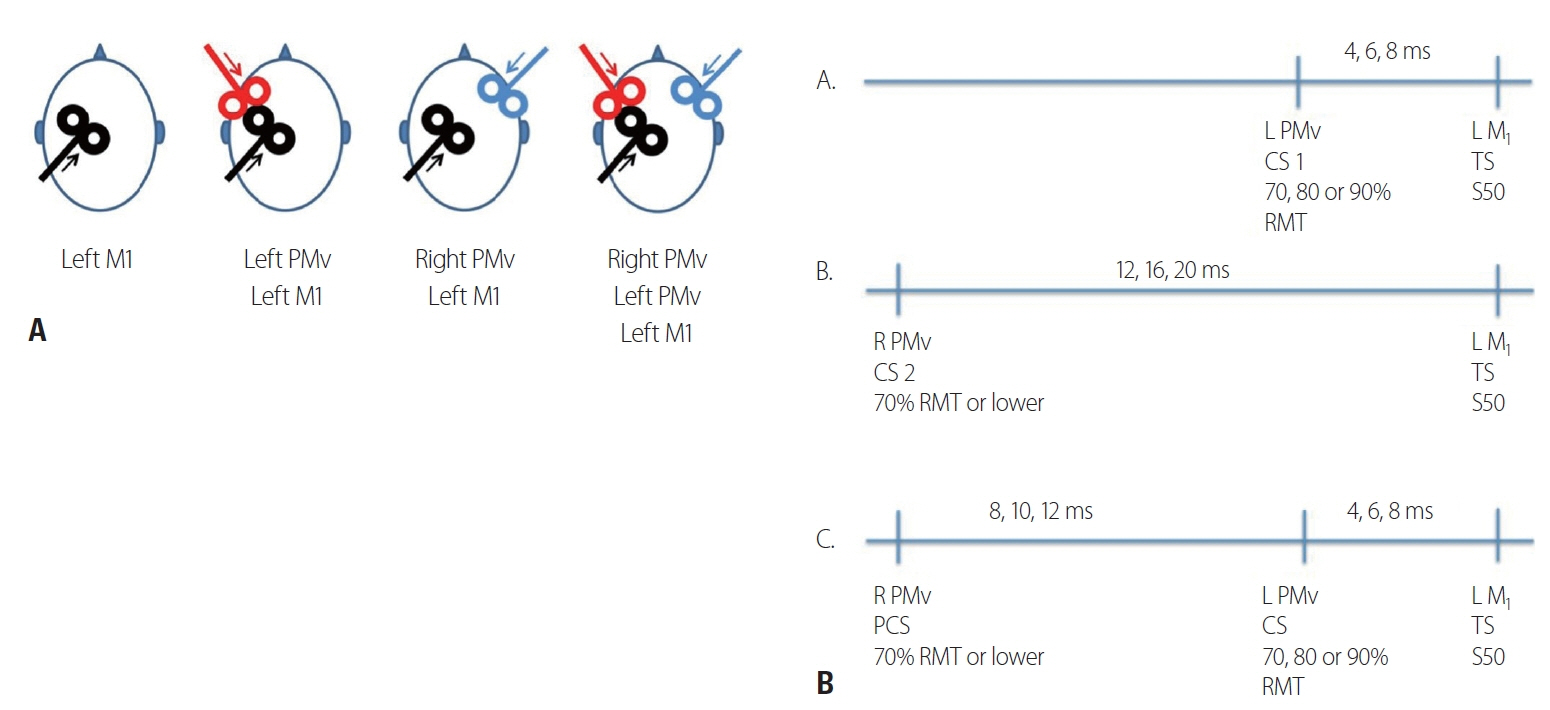
Fourteen right-handed healthy subjects (six women and eight men; mean age, 37 years; standard deviation, 14 years) completed the study. We used a three single-pulse TMS paradigm (preconditioning, conditioning and test pulse) to sequentially stimulate the right ventral premotor cortex, left ventral premotor cortex and left primary motor cortex.
Results
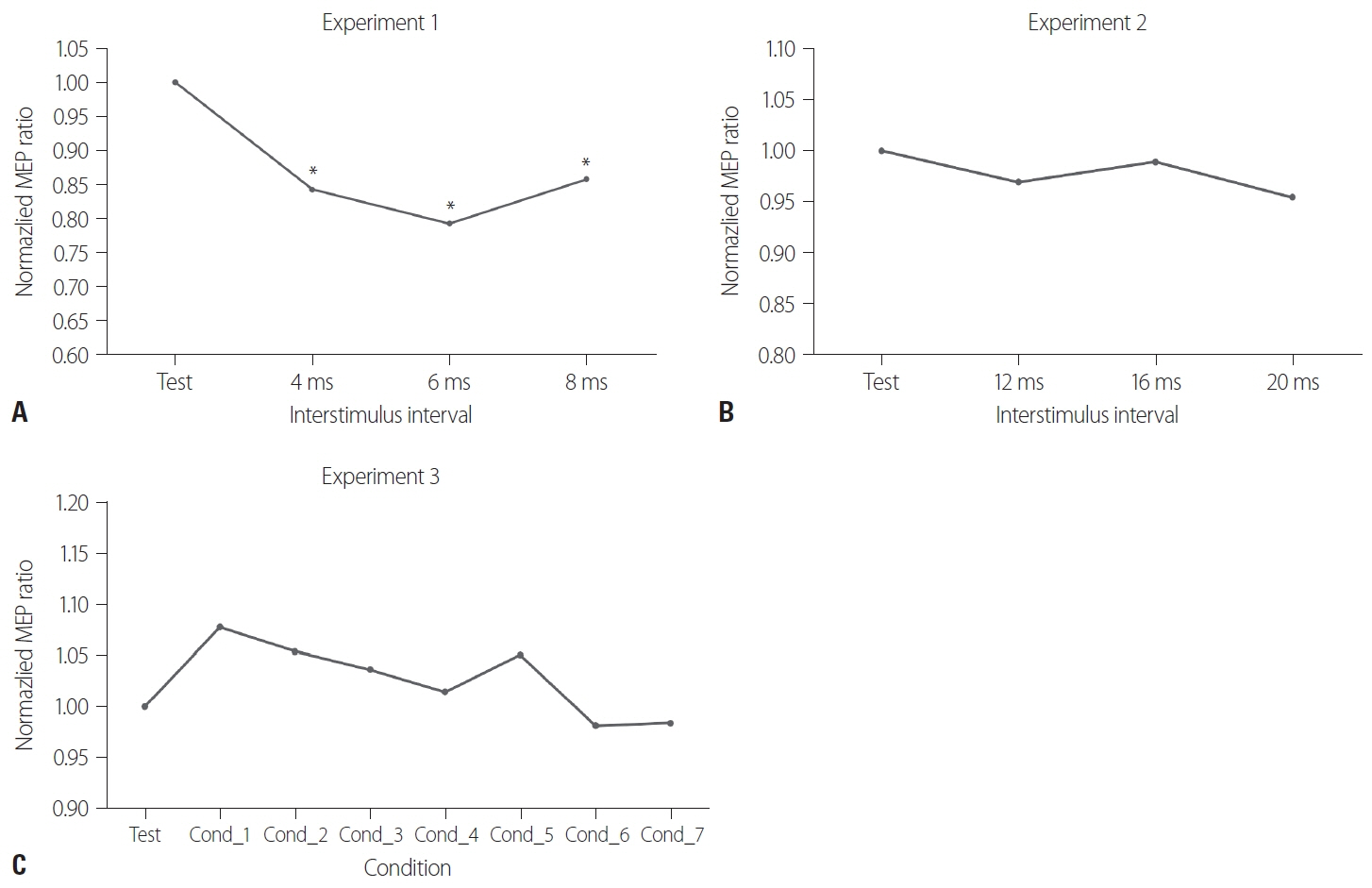
We found that in healthy subjects at rest, stimulating the contralateral ventral premotor cortex resulted in reversal of the resting premotor-to-motor inhibition.
Conclusions
Our results suggest that the contralateral ventral premotor cortex exerts an inhibitory influence on the ipsilateral ventral premotor cortex, which may be a component of bi-hemispheric control of manual tasks. This is the first study to evaluate the functional connectivity between the bilateral ventral premotor cortices.
Keyword
Figure
Reference
-
1. Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006; 26:2260–2268.
Article2. Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008; 586:2735–2742.
Article3. Arai N, Lu MK, Ugawa Y, Ziemann U. Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp Brain Res. 2012; 220:79–87.
Article4. Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007; 27:6815–6822.
Article5. Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007; 55:187–199.
Article6. Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, et al. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009; 587:4281–4292.
Article7. Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995; 37:703–713.
Article8. Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992; 453:525–546.
Article9. Cattaneo L, Barchiesi G. Transcranial magnetic mapping of the short-latency modulations of corticospinal activity from the ipsilateral hemisphere during rest. Front Neural Circuits. 2011; 5:14.
Article10. Ziluk A, Premji A, Nelson AJ. Functional connectivity from area 5 to primary motor cortex via paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2010; 484:81–85.
Article11. Bäumer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, et al. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest--a bifocal TMS study. Clin Neurophysiol. 2009; 120:1724–1731.
Article12. Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009; 45:1050–1057.
Article13. Boussaoud D, Tanné-Gariépy J, Wannier T, Rouiller EM. Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: a multiple retrograde tracing study. BMC Neurosci. 2005; 6:67.
Article14. Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci. 2003; 18:775–788.
Article15. Bencivenga F, Tullo MG, Sulpizio V, Galati G. Interhemispheric interplay between the left and right premotor cortex during grasping as assessed by dynamic causal modelling. Sci Rep. 2023; 13:4958.16. Denyer R, Greeley B, Greenhouse I, Boyd LA. Interhemispheric inhibition between dorsal premotor and primary motor cortices is released during preparation of unimanual but not bimanual movements. Eur J Neurosci. 2024; 59:415–433.
Article17. Unger RH, Lowe MJ, Beall EB, Bethoux F, Jones SE, Machado AG, et al. Stimulation of the premotor cortex enhances interhemispheric functional connectivity in association with upper limb motor recovery in moderate-to-severe chronic stroke. Brain Connect. 2023; 13:453–463.
Article18. Pereira J, Direito B, Sayal A, Ferreira C, Castelo-Branco M. Self-modulation of premotor cortex interhemispheric connectivity in a real-time functional magnetic resonance imaging neurofeedback study using an adaptive approach. Brain Connect. 2019; 9:662–672.
Article19. Dancause N, Barbay S, Frost SB, Plautz EJ, Popescu M, Dixon PM, et al. Topographically divergent and convergent connectivity between premotor and primary motor cortex. Cereb Cortex. 2006; 16:1057–1068.
Article20. Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999; 11:3276–3286.
Article21. Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006; 32:989–994.
Article22. Jarbo K, Verstynen T, Schneider W. In vivo quantification of global connectivity in the human corpus callosum. NeuroImage. 2012; 59:1988–1996.
Article23. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004; 55:400–409.
Article24. Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005; 28:940–946.
Article25. Boroojerdi B, Hungs M, Mull M, Töpper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1998; 109:230–237.
Article26. Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R. Impaired interhemispheric inhibition in writer’s cramp. Neurology. 2010; 75:441–447.
Article27. Li JY, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE, et al. Interhemispheric and ipsilateral connections in Parkinson’s disease: relation to mirror movements. Mov Disord. 2007; 22:813–821.
Article28. Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex. 2008; 18:638–647.
Article29. Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia. Eur J Neurosci. 2012; 35:478–785.
Article30. Koch G, Schneider S, Bäumer T, Franca M, Münchau A, Cheeran B, et al. Altered dorsal premotor-motor interhemispheric pathway activity in focal arm dystonia. Mov Disord. 2008; 23:660–668.
Article31. Bhasin A, Padma Srivastava MV, Kumaran SS, Bhatia R, Mohanty S. Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol India. 2012; 60:570–576.
Article32. Manthey S, Schubotz RI, von Cramon DY. Premotor cortex in observing erroneous action: an fMRI study. Brain Res Cogn Brain Res. 2003; 15:296–307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Premotor Cortex Stimulation on Motor Learning in Basal Ganglial Hemorrhage Patients
- New Onset Obsessive Compulsive Disorder Following High Frequency Repetitive Transcranial Magnetic Stimulation over Left Dorsolateral Prefrontal Cortex for Treatment of Negative Symptoms in a Patient with Schizophrenia
- Therapeutic Application of Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation in Depression
- Transcranial Magnetic Stimulation in Neuropsychiatry
- Motor Evoked Potentials by Transcranial Magnetic Stimulation