Intest Res.
2024 Oct;22(4):453-463. 10.5217/ir.2024.00044.
Predictors of histologic remission in patients with biologic-naïve, moderate-to-severe ulcerative colitis treated with first-line biologic agents and small-molecule drugs: a single-center, retrospective cohort study
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2560295
- DOI: http://doi.org/10.5217/ir.2024.00044
Abstract
- Background/Aims
The prevalence and incidence of ulcerative colitis (UC) in Korea is increasing. Each patient has a different disease course and treatment response. Recently, with the development of biologic agents, histological remission has become a treatment goal. In this study, we aimed to identify the predictors of histological remission after first-line biologic agent treatment in patients with biologic agent-naïve UC.
Methods
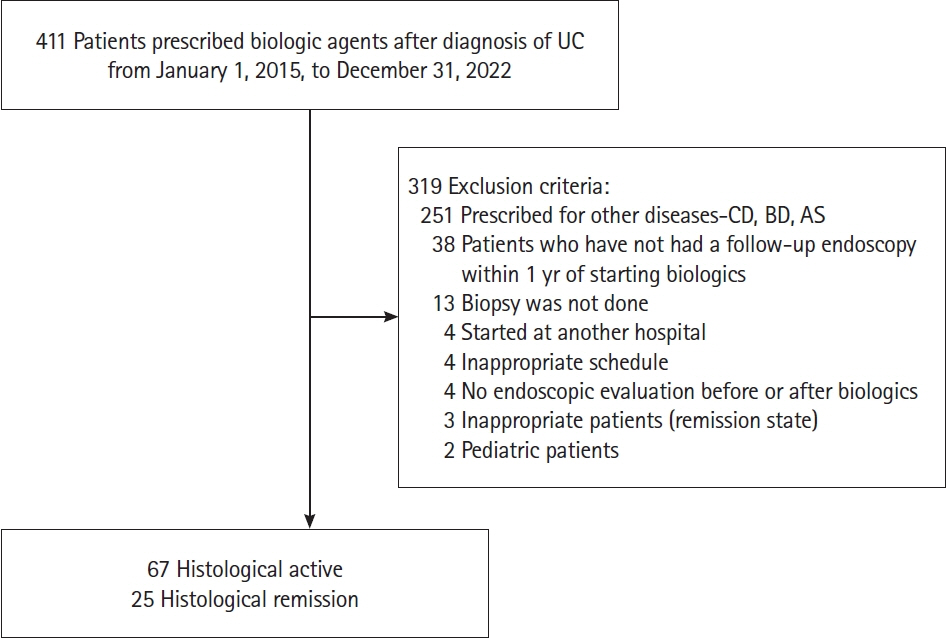
We retrospectively analyzed the medical records of 92 patients who had been diagnosed with UC and treated with first-line biologic agent treatment at our center, between 2015 and 2022. The clinical characteristics, laboratory test results, and endoscopic and biopsy findings were analyzed. Histological remission was defined as the absence of cryptitis, crypt abscesses, and inflammatory cells on histology. Univariate and multivariate logistic regression analyses were performed to identify the predictors of histological remission after first-line treatment.
Results
Of the total 92 patients, 25 (27.2%) achieved histological remission. Each cohort had a varied body mass index (BMI) distribution, with a statistically significant overweight ratio, as defined by the Asian-Pacific BMI category of 23–25 kg/m2, of 48.0% in the histological remission cohort (P= 0.026). A causal correlation between the overweight category and histological remission was confirmed (odds ratio, 3.883; 95% confidence interval, 1.141–13.212; P= 0.030).
Conclusions
We confirmed that the overweight category was a predictor of histological remission after first-line treatment with a biological agent. However, as BMI does not account for skeletal muscle mass, future studies are required to confirm the correlation between skeletal muscle mass and histological remission.
Figure
Reference
-
1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article2. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018; 16:343–356.3. Cha JM, Park SH, Rhee KH, et al. Long-term prognosis of ulcerative colitis and its temporal changes between 1986 and 2015 in a population-based cohort in the Songpa-Kangdong district of Seoul, Korea. Gut. 2020; 69:1432–1440.
Article4. Lee JW, Eun CS. Inflammatory bowel disease in Korea: epidemiology and pathophysiology. Korean J Intern Med. 2022; 37:885–894.
Article5. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140:1785–1794.
Article6. Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014; 20:3255–3264.
Article7. Quetglas EG, Mujagic Z, Wigge S, et al. Update on pathogenesis and predictors of response of therapeutic strategies used in inflammatory bowel disease. World J Gastroenterol. 2015; 21:12519–12543.8. Lee CH, Koh SJ, Radi ZA, Habtezion A. Animal models of inflammatory bowel disease: novel experiments for revealing pathogenesis of colitis, fibrosis, and colitis-associated colon cancer. Intest Res. 2023; 21:295–305.
Article9. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022; 16:2–17.
Article10. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.
Article11. Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022; 21:103017.
Article12. Na SY, Choi CH, Song EM, et al. Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis. Intest Res. 2023; 21:61–87.13. Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021; 12:651415.
Article14. Song HY, Seo GS. Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies. J Korean Med Assoc. 2021; 64:605–613.
Article15. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article16. Magro F, Lopes SI, Lopes J, et al. Histological outcomes and predictive value of faecal markers in moderately to severely active ulcerative colitis patients receiving infliximab. J Crohns Colitis. 2016; 10:1407–1416.
Article17. Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014; 12:929–934.18. Yoon H, Jangi S, Dulai PS, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology. 2020; 159:1262–1275.
Article19. Chateau T, Feakins R, Marchal-Bressenot A, Magro F, Danese S, Peyrin-Biroulet L. Histological remission in ulcerative colitis: under the microscope is the cure. Am J Gastroenterol. 2020; 115:179–189.
Article20. O’Toole A, Moss AC. Optimizing biologic agents in ulcerative colitis and Crohn’s disease. Curr Gastroenterol Rep. 2015; 17:32.
Article21. Zhao M, Gönczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021; 15:1573–1587.22. Kim JW, Lee CK, Lee JK, et al. Long-term evolution of direct healthcare costs for inflammatory bowel diseases: a population-based study (2006-2015). Scand J Gastroenterol. 2019; 54:419–426.23. Song EM, Yang SK. Natural history of inflammatory bowel disease: a comparison between the East and the West. Intest Res. 2022; 20:418–430.
Article24. Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020; 14:694–709.
Article25. Zammarchi I, Lanzarotto F, Cannatelli R, et al. Elderly-onset vs adult-onset ulcerative colitis: a different natural history? BMC Gastroenterol. 2020; 20:147.
Article26. World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000.27. Gheorghe C, Cotruta B, Iacob R, Becheanu G, Dumbrava M, Gheorghe L. Endomicroscopy for assessing mucosal healing in patients with ulcerative colitis. J Gastrointestin Liver Dis. 2011; 20:423–426.28. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. Firstand second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020; 18:2179–2191.29. Lu X, Jarrett J, Sadler S, Tan M, Dennis J, Jairath V. Comparative efficacy of advanced treatments in biologic-naïve or biologic-experienced patients with ulcerative colitis: a systematic review and network meta-analysis. Int J Clin Pharm. 2023; 45:330–341.
Article30. Battat R, Duijvestein M, Guizzetti L, et al. Histologic healing rates of medical therapies for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. Am J Gastroenterol. 2019; 114:733–745.
Article31. Guerbau L, Gerard R, Duveau N, et al. Patients with Crohn’s disease with high body mass index present more frequent and rapid loss of response to infliximab. Inflamm Bowel Dis. 2017; 23:1853–1859.
Article32. Kim JH, Oh CM, Yoo JH. Obesity and novel management of inflammatory bowel disease. World J Gastroenterol. 2023; 29:1779–1794.
Article33. Knibbe CA, Brill MJ, van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol. 2015; 55:149–167.34. Karaskova E, Velganova-Veghova M, Geryk M, Foltenova H, Kucerova V, Karasek D. Role of adipose tissue in inflammatory bowel disease. Int J Mol Sci. 2021; 22:4226.35. Teixeira LG, Leonel AJ, Aguilar EC, et al. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011; 10:204.36. Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016; 22:7868–7881.37. Nishikawa H, Nakamura S, Miyazaki T, et al. Inflammatory bowel disease and sarcopenia: its mechanism and clinical importance. J Clin Med. 2021; 10:4214.38. Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017; 96:10–15.39. Fatani H, Olaru A, Stevenson R, et al. Systematic review of sarcopenia in inflammatory bowel disease. Clin Nutr. 2023; 42:1276–1291.40. Liu S, Ding X, Maggiore G, et al. Sarcopenia is associated with poor clinical outcomes in patients with inflammatory bowel disease: a prospective cohort study. Ann Transl Med. 2022; 10:367.
Article41. Cushing KC, Kordbacheh H, Gee MS, Kambadakone A, Ananthakrishnan AN. Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients. J Crohns Colitis. 2018; 12:1036–1041.42. Bilski J, Brzozowski B, Mazur-Bialy A, Sliwowski Z, Brzozowski T. The role of physical exercise in inflammatory bowel disease. Biomed Res Int. 2014; 2014:429031.43. Cook MD, Martin SA, Williams C, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013; 33:46–56.44. Hoffman-Goetz L, Pervaiz N, Packer N, Guan J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav Immun. 2010; 24:1105–1115.
Article45. Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev. 2018; 17:53–72.46. Groothof D, Post A, Polinder-Bos HA, Hazenberg BP, Gans RO, Bakker SJ. Muscle mass versus body mass index as predictor of adverse outcome. J Cachexia Sarcopenia Muscle. 2021; 12:517–518.
Article47. Pan L, Xie W, Fu X, et al. Inflammation and sarcopenia: a focus on circulating inflammatory cytokines. Exp Gerontol. 2021; 154:111544.48. Rozich JJ, Holmer A, Singh S. Effect of lifestyle factors on outcomes in patients with inflammatory bowel diseases. Am J Gastroenterol. 2020; 115:832–840.49. Feng Y, Feng W, Xu M, et al. Sarcopenia and treatment failure in inflammatory bowel disease: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2024; 116:68–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Long-term Outcomes of Infliximab versus Adalimumab Treatment in Biologic-Naïve Patients with Ulcerative Colitis
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies
- Comparison of Treatment Guidelines for Ulcerative Colitis: Role of Biologics
- Advancements in the Management of Moderate-to-Severe Ulcerative Colitis: A Revised 2023 Korean Treatment Guidelines
- Treatment of inflammatory bowel diseases: focusing on 5-aminosalicylates and immunomodulators