Intest Res.
2024 Oct;22(4):414-427. 10.5217/ir.2024.00001.
Complex dichotomous links of nonalcoholic fatty liver disease and inflammatory bowel disease: exploring risks, mechanisms, and management modalities
- Affiliations
-
- 1Department of Medicine, Dayanand Medical College, Ludhiana, India
- 2Department of Medicine, Government Medical College Amritsar, Amritsar, India
- 3Department of Medicine, Cape Fear Valley Medical Center, Fayetteville, NC, USA
- 4Department of Medicine, Nazareth Hospital, Philadelphia, PA, USA
- 5Department of Medicine, Metropolitan Hospital Center, New York, NY, USA
- 6Department of Medicine, University College of Medical Sciences, New Delhi, India
- 7Amity Regional High School, Woodbridge, CT, USA
- 8Department of Medicine, Penn State Milton S. Hershey Medical Center, Hershey, PA, USA
- KMID: 2560292
- DOI: http://doi.org/10.5217/ir.2024.00001
Abstract
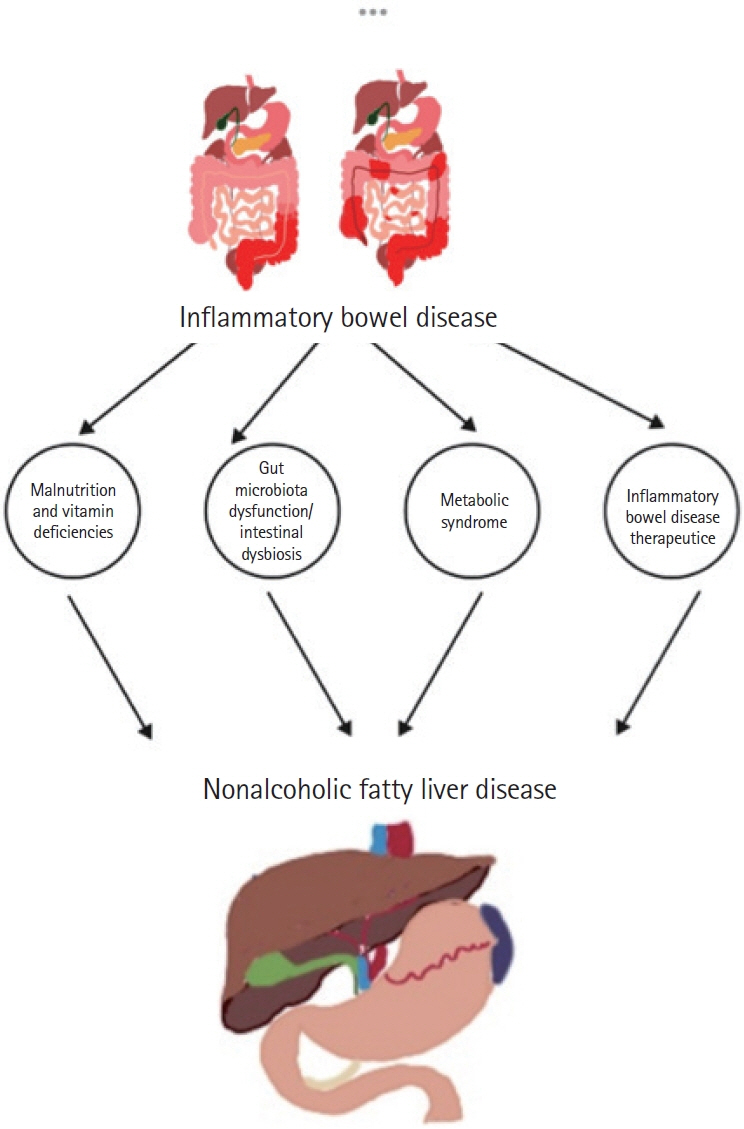
- Nonalcoholic fatty liver disease (NAFLD) has been shown to be linked to inflammatory bowel disease (IBD) due to established risk factors such as obesity, age, and type 2 diabetes in numerous studies. However, alternative research suggests that factors related to IBD, such as disease activity, duration, and drug-induced toxicity, can contribute to NAFLD. Recent research findings suggest IBD relapses are correlated with dysbiosis, mucosal damage, and an increase in cytokines. In contrast, remission periods are characterized by reduced metabolic risk factors. There is a dichotomy evident in the associations between NAFLD and IBD during relapses and remissions. This warrants a nuanced understanding of the diverse influences on disease manifestation and progression. It is possible to provide a holistic approach to care for patients with IBD by emphasizing the interdependence between metabolic and inflammatory disorders.
Keyword
Figure
Reference
-
1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015; 12:720–727.2. Barnes EL, Loftus EV Jr, Kappelman MD. Effects of race and ethnicity on diagnosis and management of inflammatory bowel diseases. Gastroenterology. 2021; 160:677–689.3. Ghouri YA, Tahan V, Shen B. Secondary causes of inflammatory bowel diseases. World J Gastroenterol. 2020; 26:3998–4017.4. Fabián O, Kamaradová K. Morphology of inflammatory bowel diseases (IBD). Cesk Patol. 2022; 58:27–37.5. Magrì S, Paduano D, Chicco F, et al. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: beyond the natural history. World J Gastroenterol. 2019; 25:5676–5686.6. Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD): pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017; 49:197–211.7. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015; 62(1 Suppl):S47–S64.8. Copyright Endocrine Society. Prevalence of metabolic associated fatty liver disease is increasing [Internet]. c2023 [cited Sep 3]. https://www.endocrine.org/news-and-advocacy/newsroom/2023/endo-2023-press-friedman.9. Liu J, Ayada I, Zhang X, et al. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol. 2022; 20:e573–e582.10. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018; 15:11–20.11. Dongiovanni P, Paolini E, Corsini A, Sirtori CR, Ruscica M. Nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease diagnoses and cardiovascular diseases: from epidemiology to drug approaches. Eur J Clin Invest. 2021; 51:e13519.12. Rodriguez-Duque JC, Calleja JL, Iruzubieta P, et al. Increased risk of MAFLD and liver fibrosis in inflammatory bowel disease independent of classic metabolic risk factors. Clin Gastroenterol Hepatol. 2023; 21:406–414.13. Ampong I, Watkins A, Gutierrez-Merino J, Ikwuobe J, Griffiths HR. Dietary protein insufficiency: an important consideration in fatty liver disease? Br J Nutr. 2020; 123:601–609.14. Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic fatty liver disease and the gut-liver axis: exploring an undernutrition perspective. Gastroenterology. 2022; 162:1858–1875.15. Barchetta I, Cimini FA, Cavallo MG. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): an update. Nutrients. 2020; 12:3302.16. Saeed A, Dullaart RP, Schreuder TC, Blokzijl H, Faber KN. Disturbed vitamin A metabolism in non-alcoholic fatty liver disease (NAFLD). Nutrients. 2017; 10:29.17. Sid V, Siow YL, O K. Role of folate in nonalcoholic fatty liver disease. Can J Physiol Pharmacol. 2017; 95:1141–1148.18. Yilmaz Y, Ulukaya E, Atug O, Dolar E. Serum concentrations of human angiopoietin-like protein 3 in patients with nonalcoholic fatty liver disease: association with insulin resistance. Eur J Gastroenterol Hepatol. 2009; 21:1247–1251.19. Jarmakiewicz-Czaja S, Sokal A, Pardak P, Filip R. Glucocorticosteroids and the risk of NAFLD in inflammatory bowel disease. Can J Gastroenterol Hepatol. 2022; 2022:4344905.20. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018; 68:280–295.21. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018; 24:908–922.22. Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol. 2018; 14:452–463.23. Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015; 16:678–689.24. Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta. 2012; 1821:21–56.25. Lee YK, Park JE, Lee M, Hardwick JP. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018; 2:209–215.26. Chen G, Weiskirchen S, Weiskirchen R. Vitamin A: too good to be bad? Front Pharmacol. 2023; 14:1186336.27. Borén J, Packard CJ, Taskinen MR. The roles of ApoC-III on the metabolism of triglyceride-rich lipoproteins in humans. Front Endocrinol (Lausanne). 2020; 11:474.28. Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021; 50:101238.29. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017; 377:2063–2072.30. Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Nonalcoholic steatohepatitis: a review of its mechanism, models and medical treatments. Front Pharmacol. 2020; 11:603926.31. Kovarova M, Königsrainer I, Königsrainer A, et al. The genetic variant I148M in PNPLA3 is associated with increased hepatic retinyl-palmitate storage in humans. J Clin Endocrinol Metab. 2015; 100:E1568–E1574.32. da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. Biofactors. 2014; 40:277–283.33. Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci. 2019; 7:263–287.34. Foulds CE, Treviño LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 2017; 13:445–457.35. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017; 13:572–587.36. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016; 48:e245.37. Jones P, Lucock M, Scarlett CJ, Veysey M, Beckett EL. Folate and inflammation–links between folate and features of inflammatory conditions. J Nutr Intermed Metab. 2019; 18:100104.38. Wu P, Jia F, Zhang B, Zhang P. Risk of cardiovascular disease in inflammatory bowel disease. Exp Ther Med. 2017; 13:395–400.39. Barrington WT, Wulfridge P, Wells AE, et al. Improving metabolic health through precision dietetics in mice. Genetics. 2018; 208:399–417.40. Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013; 3:1191–1212.41. Long SL, Gahan CG, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017; 56:54–65.42. Kuna AT. Serological markers of inflammatory bowel disease. Biochem Med (Zagreb). 2013; 23:28–42.43. Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013; 7:e279–e285.44. Carr RM, Patel A, Bownik H, et al. Intestinal inflammation does not predict nonalcoholic fatty liver disease severity in inflammatory bowel disease patients. Dig Dis Sci. 2017; 62:1354–1361.45. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016; 65:1038–1048.46. Xu L, Kitade H, Ni Y, Ota T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules. 2015; 5:1563–1579.47. Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012; 18:727–735.48. Chakravarthy MV, Waddell T, Banerjee R, Guess N. Nutrition and nonalcoholic fatty liver disease: current perspectives. Gastroenterol Clin North Am. 2020; 49:63–94.49. Mazidi M, Katsiki N, Mikhailidis DP, Banach M. Adiposity may moderate the link between choline intake and non-alcoholic fatty liver disease. J Am Coll Nutr. 2019; 38:633–639.50. Chao CY, Battat R, Al Khoury A, Restellini S, Sebastiani G, Bessissow T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: a review article. World J Gastroenterol. 2016; 22:7727–7734.51. Campbell JE, Peckett AJ, D’souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol. 2011; 300:C198–C209.52. Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022; 18:540–557.53. Salehidoost R, Korbonits M. Glucose and lipid metabolism abnormalities in Cushing’s syndrome. J Neuroendocrinol. 2022; 34:e13143.54. Rahimi L, Rajpal A, Ismail-Beigi F. Glucocorticoid-induced fatty liver disease. Diabetes Metab Syndr Obes. 2020; 13:1133–1145.55. Bath RK, Brar NK, Forouhar FA, Wu GY. A review of methotrexate-associated hepatotoxicity. J Dig Dis. 2014; 15:517–524.56. Bedoui Y, Guillot X, Sélambarom J, et al. Methotrexate an old drug with new tricks. Int J Mol Sci. 2019; 20:5023.57. Hamed KM, Dighriri IM, Baomar AF, et al. Overview of methotrexate toxicity: a comprehensive literature review. Cureus. 2022; 14:e29518.58. Conway R, Carey JJ. Risk of liver disease in methotrexate treated patients. World J Hepatol. 2017; 9:1092–1100.59. Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017; 3(Suppl 1):212–232.60. Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007; 102:1518–1527.
Article61. Núñez F P, Quera R, Bay C, Castro F, Mezzano G. Drug-induced liver injury used in the treatment of inflammatory bowel disease. J Crohns Colitis. 2022; 16:1168–1176.
Article62. Calafat M, Mañosa M, Cañete F, et al. Increased risk of thiopurine-related adverse events in elderly patients with IBD. Aliment Pharmacol Ther. 2019; 50:780–788.
Article63. Becker HE, Demers K, Derijks LJ, Jonkers DM, Penders J. Current evidence and clinical relevance of drug-microbiota interactions in inflammatory bowel disease. Front Microbiol. 2023; 14:1107976.64. Dean L. Azathioprine therapy and TPMT and NUDT15 genotype. In: Pratt VM, Scott SA, Pirmohamed M, et al., eds. Medical genetics summaries. Bethesda: National Center for Biotechnology Information (US); 2012. https://www.ncbi.nlm.nih.gov/books/NBK100661/.65. Grau T, Bonet A, Rubio M, et al. Liver dysfunction associated with artificial nutrition in critically ill patients. Crit Care. 2007; 11:R10.66. Martínez-Domínguez SJ, García-Mateo S, Laredo V, et al. Liver fibrosis in non-alcoholic fatty liver disease and progression to hepatocellular carcinoma in patients with inflammatory bowel disease: a systematic review. Cancers (Basel). 2023; 15:3367.
Article67. Adams LC, Lübbe F, Bressem K, Wagner M, Hamm B, Makowski MR. Non-alcoholic fatty liver disease in underweight patients with inflammatory bowel disease: a case-control study. PLoS One. 2018; 13:e0206450.68. Onwuzo S, Boustany A, Saleh M, et al. Increased risk of non-alcoholic steatohepatitis in patients with inflammatory bowel disease: a population-based study. Cureus. 2023; 15:e35854.69. Abenavoli L, Giubilei L, Procopio AC, et al. Gut microbiota in non-alcoholic fatty liver disease patients with inflammatory bowel diseases: a complex interplay. Nutrients. 2022; 14:5323.
Article70. Singh B, Khan AA, Anamika F, Munjal R, Munjal J, Jain R. Red meat consumption and its relationship with cardiovascular health: a review of pathophysiology and literature. Cardiol Rev. 2023; Jun. 26. [Epub]. https://doi.org/10.1097/CRD.0000000000000575.71. Pan X, Wen SW, Kaminga AC, Liu A. Gut metabolites and inflammation factors in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sci Rep. 2020; 10:8848.72. Kim G, Lee SE, Lee YB, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7-year longitudinal study. Hepatology. 2018; 68:1755–1768.
Article73. Wijarnpreecha K, Kim D, Raymond P, Scribani M, Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019; 31:1121–1128.
Article74. Dhaliwal A, Quinlan JI, Overthrow K, et al. Sarcopenia in inflammatory bowel disease: a narrative overview. Nutrients. 2021; 13:656.
Article75. Principi M, Iannone A, Losurdo G, et al. Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis. 2018; 24:1589–1596.76. Saroli Palumbo C, Restellini S, Chao CY, et al. Screening for nonalcoholic fatty liver disease in inflammatory bowel diseases: a cohort study using transient elastography. Inflamm Bowel Dis. 2019; 25:124–133.
Article77. Sartini A, Gitto S, Bianchini M, et al. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018; 9:87.
Article78. Kodali A, Okoye C, Klein D, et al. Crohn’s disease is a greater risk factor for nonalcoholic fatty liver disease compared to ulcerative colitis: a systematic review. Cureus. 2023; 15:e42995.
Article79. Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022; 22:63.
Article80. da Silva LC, de Oliveira JT, Tochetto S, de Oliveira CP, Sigrist R, Chammas MC. Ultrasound elastography in patients with fatty liver disease. Radiol Bras. 2020; 53:47–55.81. Zaman CF, Sultana J, Dey P, et al. A multidisciplinary approach and current perspective of nonalcoholic fatty liver disease: a systematic review. Cureus. 2022; 14:e29657.
Article82. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015; 149:367–378.
Article83. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010; 362:1675–1685.
Article84. Ni Y, Qian L, Siliceo SL, et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023; 35:1530–1547.
Article85. Tang KT, Dufour JF, Chen PH, Hernaez R, Hutfless S. Antitumour necrosis factor-α agents and development of new-onset cirrhosis or non-alcoholic fatty liver disease: a retrospective cohort. BMJ Open Gastroenterol. 2020; 7:e000349.86. Lapumnuaypol K, Kanjanahattakij N, Pisarcik D, Thongprayoon C, Wijarnpreecha K, Cheungpasitporn W. Effects of inflammatory bowel disease treatment on the risk of nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018; 30:854–860.
Article87. Boustany A, Rahhal R, Mitri J, et al. The impact of nonalcoholic fatty liver disease on inflammatory bowel disease-related hospitalization outcomes: a systematic review. Eur J Gastroenterol Hepatol. 2023; 35:1067–1074.
Article88. Beard JA, Click BH. The burden of cost in inflammatory bowel disease: a medical economic perspective. Curr Opin Gastroenterol. 2020; 36:310–316.
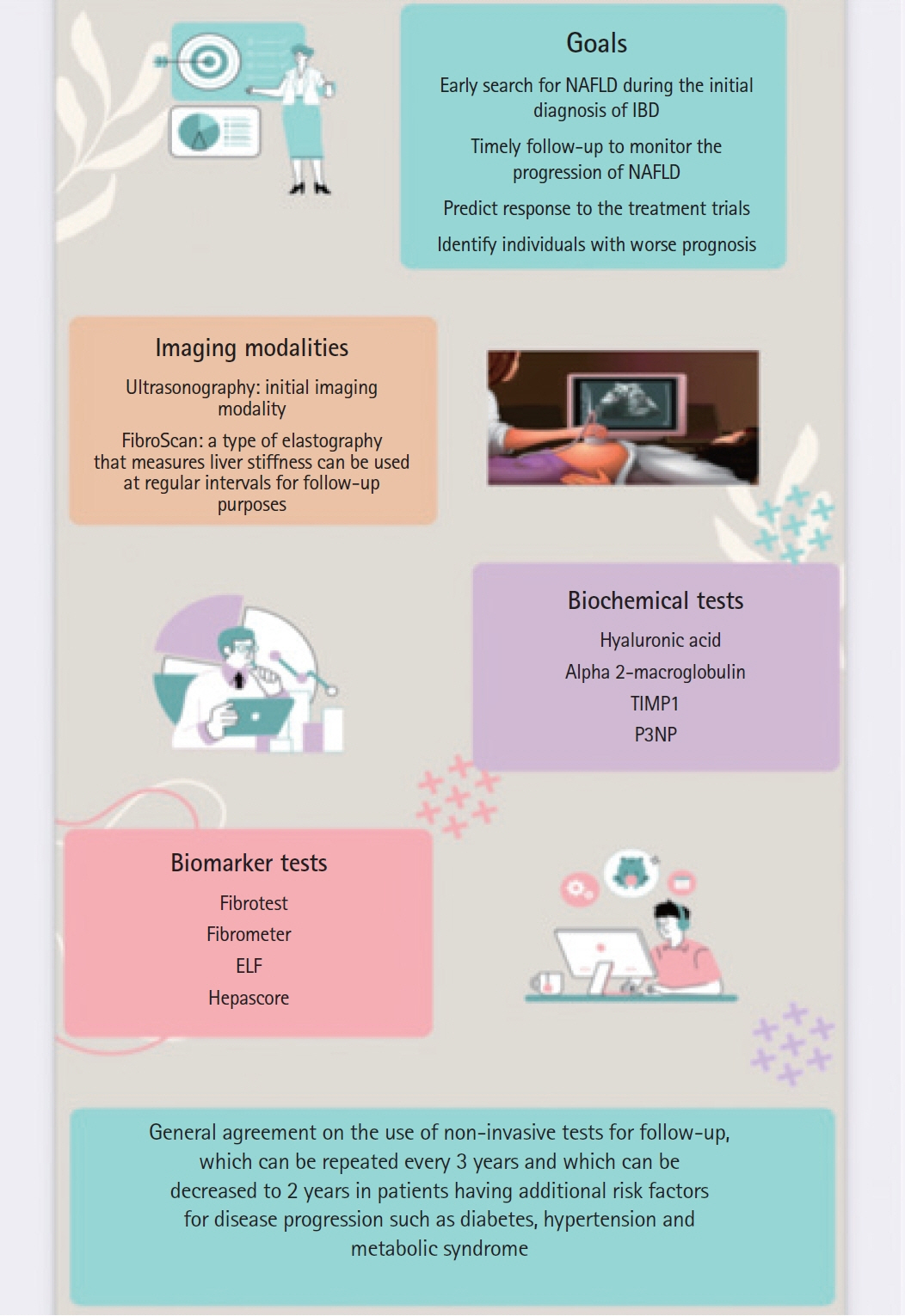
Article89. Boursier J, Guillaume M, Bouzbib C, et al. Non-invasive diagnosis and follow-up of non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2022; 46:101769.
Article90. Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016; 65:570–578.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elucidating the association between nonalcoholic fatty liver disease and incidence of inflammatory bowel disease: a focus on systemic inflammation
- The crosstalk between insulin resistance and nonalcoholic fatty liver disease/metabolic dysfunction-associated fatty liver disease: a culprit or a consequence?
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- The diagnosis of nonalcoholic fatty liver disease
- Nonalcoholic fatty liver disease: pathogenesis and treatment